Organic molecules with molecular asymmetry, showing chirality without a stereogenic atom, are interesting for applications in e.g., asymmetric catalysis or chirality recognition. In contrast to molecules with a stereogenic carbon atom, chirality based on molecular asymmetry is, in theory, switchable by a conformational change without breaking and reforming a chemical bond.
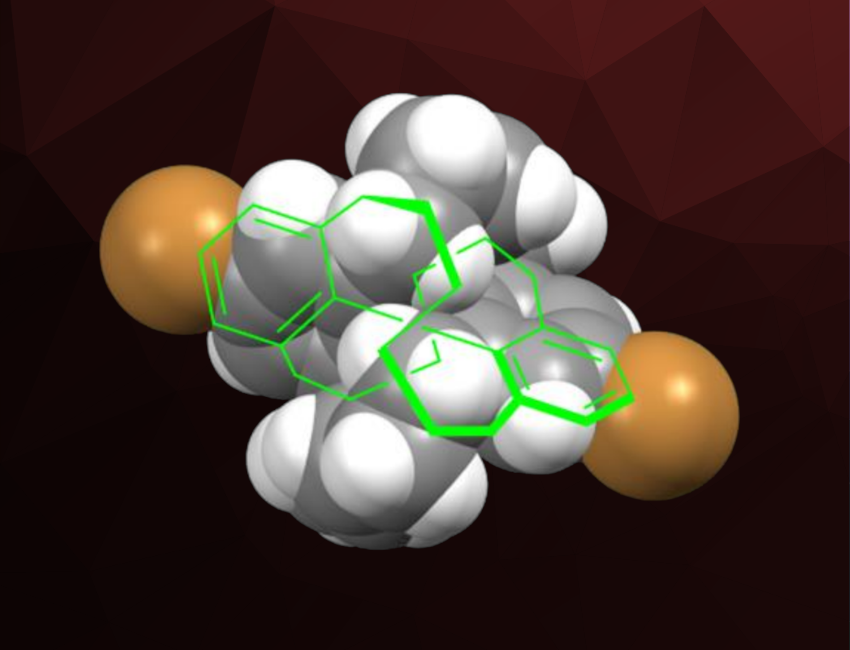
Atsunori Mori, Kobe University, Japan, and colleagues have synthesized biphenyl derivatives in which two benzene rings are connected by two winding chains, resulting in molecular asymmetry (example pictured). They started from pyrene, which was reacted with NaIO4 in the presence of RuCl3 to obtain a biphenyl tetracarboxylic acid. This intermediate was reduced to the corresponding tetraol. Alternatively, a brominated analogue was obtained via ozonolysis, bromination, and reduction.
The resulting tetraols were functionalized via halogenation and a reaction with an allyl Grignard reagent to obtain precursors for ring-closing metathesis. The ring-closing metathesis then closed two “chains” connecting the aromatic rings. For the brominated derivative, the team was able to obtain an X-ray crystal structure, confirming the formation of the desired product as a racemate. The researchers also used a nosyl annulation reaction (nosyl: 2-nitrobenzenesulfonyl) to obtain a similar doubly ring-closed product with nitrogen atoms in the connecting chains.
The team calls the type of chirality in the ring-closed products “double winding vine-shaped” molecular asymmetry. Density functional theory (DFT) calculations suggest that the racemization of the double winding vine biphenyls would hardly take place.
- Double winding vine‐shaped biphenyl with molecular asymmetry. Synthesis, structure, and properties,
Atsunori Mori, Aruto Maruka, Kohei Tabuchi, Kentaro Okano, Masaki Horie,
Eur. J. Org. Chem. 2024.
https://doi.org/10.1002/ejoc.202400192