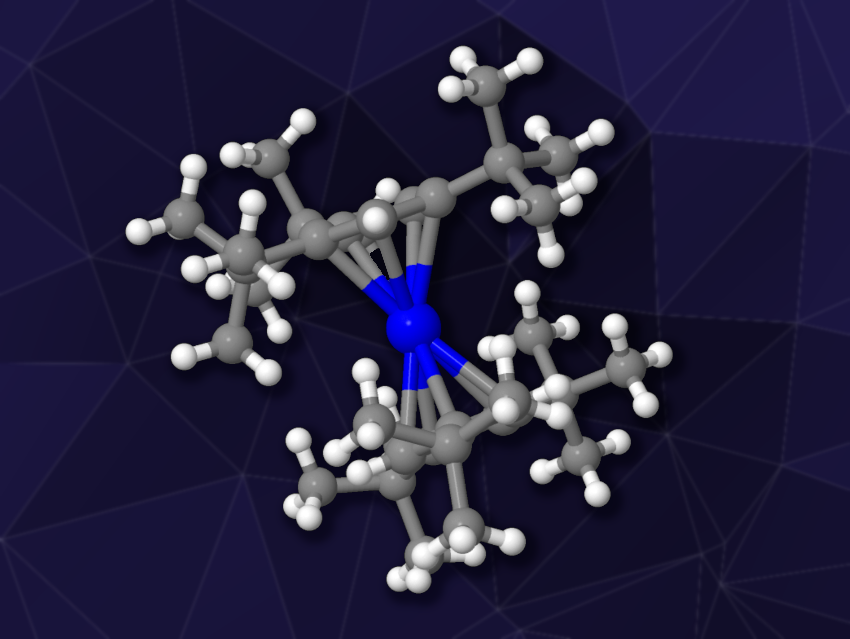
Ferrocene is a sandwich complex consisting of two cyclopentadienyl rings bound to an iron atom. Other metallocenes are also known, but bis(cyclopentadienyl) complexes of scandium, i.e., Sc(II) scandocenes, had remained elusive so far. There have been reports of a heteroatomic scandium metallocene, but this complex was too reactive for crystallographic characterization.
Filipp Furche, William J. Evans, University of California, Irvine, USA, and colleagues have synthesized the first crystallographically characterizable bis(cyclopentadienyl) Sc(II) complex, (C5H2tBu3)2Sc (pictured), which has tri-tert-butyl-substituted cyclopentadienyl ligands for steric protection. The team first prepared the iodide (C5H2tBu3)2ScI as a precursor from KC5H2tBu3 and ScI3.
This precursor was then reduced using KC8 in hexane at room temperature under an Ar atmosphere, giving the desired (C5H2tBu3)2Sc in a yield of about 75 %. The product was recrystallized and characterized using X-ray diffraction. The team found that the synthesized complex is, indeed, a scandocene and thermally stable. The cyclopentadienyl rings are not parallel as in ferrocene, but form a Cp–Sc–Cp angle of 170.6°. Of the six tert-butyl ligands, four are arranged in a staggered manner and two are eclipsed.
The researchers also reduced the precursor C5H3(SiMe3)2ScI using KC8 in diethylether in the presence of 2.2.2-cryptand and obtained the complex salt [K(crypt)]{[C5H3(SiMe3)2]2ScI}. X-ray crystallography showed that the anion in this compound is a bis(cyclopentadienyl) Sc(II) complex that retains an iodide ligand. However, this product is thermally unstable. Overall, the work extends the well-known bis(cyclopentadienyl) chemistry of other transition metals to scandium.
- Synthesis of Crystallographically Characterizable Bis(cyclopentadienyl) Sc(II) Complexes: (C5H2tBu3)2Sc and {[C5H3(SiMe3)2]2ScI}1–,
Joshua D. Queen, Lauren M. Anderson-Sanchez, Cary R. Stennett, Ahmadreza Rajabi, Joseph W. Ziller, Filipp Furche, William J. Evans,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.3c11922