Can we harvest drinking water directly from the air around us? Heat waves and droughts are gaining in frequency and intensity, and freshwater scarcity is becoming an increasingly urgent problem in many places. Instead of digging ever deeper wells and transporting water over long distances, converting atmospheric vapor to liquid water could be a promising alternative solution. When investigating practical technologies to achieve this, however, energy input and materials design represent significant challenges.
Using the Dew Point – Nature’s Way
The most obvious method for harvesting moisture from the air is using the dew point. The dew point is the temperature at which air, containing a certain amount of humidity, becomes saturated with moisture. At this point, the moisture can condense out of the air, e.g., onto a surface. For example, when the air temperature is 25 °C, and the relative humidity is 35 %, the dew point is 8.5 °C. If the 25 °C warm air has 65 % relative humidity, the dew point is 18 °C.
|
|
|
Figure 1. Dew droplets on a cactus. |
Many species in Nature have evolved to exploit this relationship to trap moisture using diurnal temperature fluctuations. To give some examples, tree frogs in tropical Australia, cooled down during the night, crawl into warm, confined places where the air holds more humidity [1,2]. Here, water vapor condenses on the frog’s cool skin, wetting it. Desert cactuses let droplets condense at the tops of their spines (see Fig. 1), from where they are guided to the stem in a channel system, exploiting capillary and other physical forces [2,3].
Combining Hydrophilic and Hydrophobic Structures
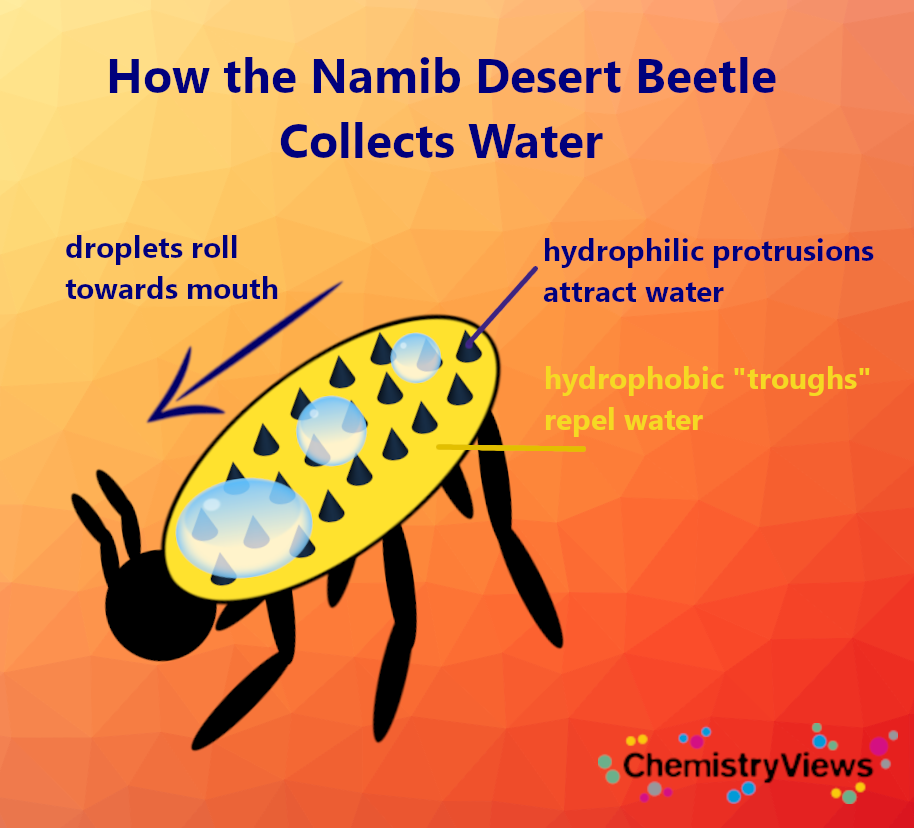
The Namib desert beetle (see Fig. 2), native to one of the most arid areas of the world, the Namib Desert in southern Africa, adds a chemical component to its water-collection design. It uses hydrophilic protrusions on its shell as sites for droplet nucleation and growth, while wax-coated troughs cause the condensing water droplets to be attracted toward the top of the protrusions. Finally, it carries a whole drop on its back, like a backpack, from which water is funneled into its mouth [4].
|
|
|
Figure 2. How the Namib desert beetle collects water. |
Humans have also mimicked the approach found in nature. Throughout human history, societies have used two- and three-dimensional structures such as plates, nets, or rods to trap fog and mist droplets (see Fig. 3). As easy as it sounds, there are obstacles to overcome that lower the yields. For example, wind direction is important because a large volume of air needs to fall on the surfaces. The incoming droplets forming also need to quickly run down the structure to fill water tanks. Besides the usual steel meshes and rods, modern nylon and polypropylene nets and films allow low contact angles so that droplets can slide down quickly.
|
|
|
Figure 3. Mist catcher (Source: SuSanA Secretariat, wikimedia commons, CC BY 2.0). |
Heat Exchanger Technologies – Independent of the Time of Day
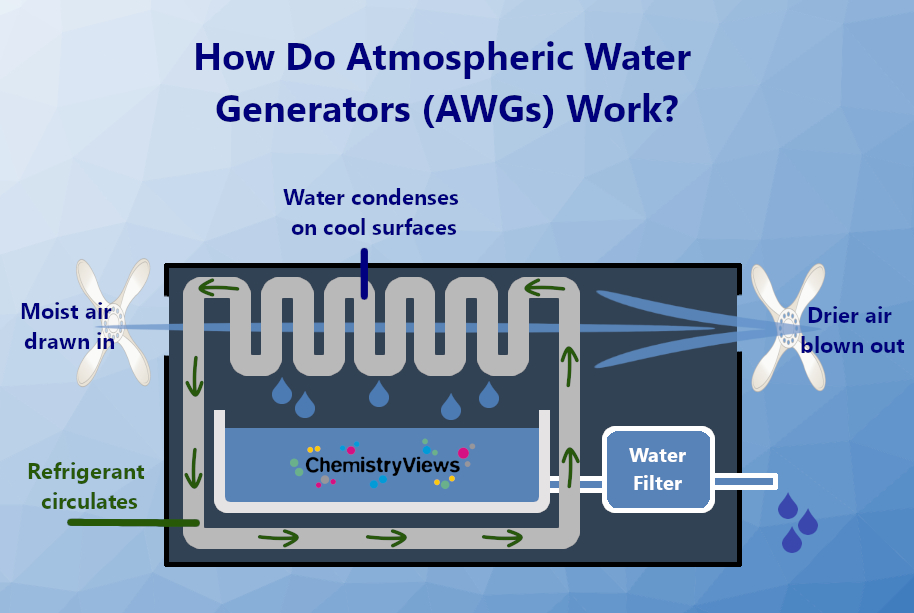
Electricity-driven heat exchangers, called atmospheric water generators (AWGs), remove the dependency of water collection on the weather or time of day. An AWG device draws in atmospheric air, usually using a fan, and guides it over cooled plates where water vapor condenses into liquid water (see Fig. 4). This water is collected, filtered, and sometimes supplemented with minerals to obtain healthy drinking water.
|
|
|
Figure 4. How Do Atmospheric Water Generators (AWGs) Work? |
AWGs require energy to work, so it is always necessary to have a charged battery, a power socket, or a solar panel nearby. The GENius Technology from Watergen, Israel, for example, claims on its website that its portable, mobile apparatus produces up to one liter of liquid water in an hour using 350 W of power [5]. This is the amount of electric power you need for 15 minutes of hair drying; it can be provided by a modern solar module of about 1.5 m2. Home-based AWGs produce 1–20 L of water per day. On a commercial scale, the production can be 1000 times higher.
However, yields decrease and energy costs increase exponentially the drier the air is. AWGs are designed to work at a relative humidity of 30 % or more and moderate temperatures. To find systems that can harvest atmospheric water virtually anywhere and at any time while using as little energy as possible, alternative technologies are needed.
Brine-Based Solutions
An entirely different way to harvest water from the atmosphere is the use of hygroscopic salts. Deliquescent salts, such as calcium chloride, capture water molecules passively through the hydration process, which involves chemical and physical absorption. (If a hygroscopic substance absorbs so much moisture that it dissolves, the substance is called deliquescent.) They form highly concentrated solutions that attract more moisture from the environment.
Researchers at the Fraunhofer Institute for Interfacial Engineering and Biotechnology, Germany, have developed a brine-based atmospheric water harvester (AWH) where a highly concentrated salt solution trickles down a tower while taking up moisture from the air [7–9]. The diluted brine is then vacuum-distilled (the vacuum reduces the evaporation temperature) and recycled into the system. Solar panels provide the energy required.
Tamed Salts
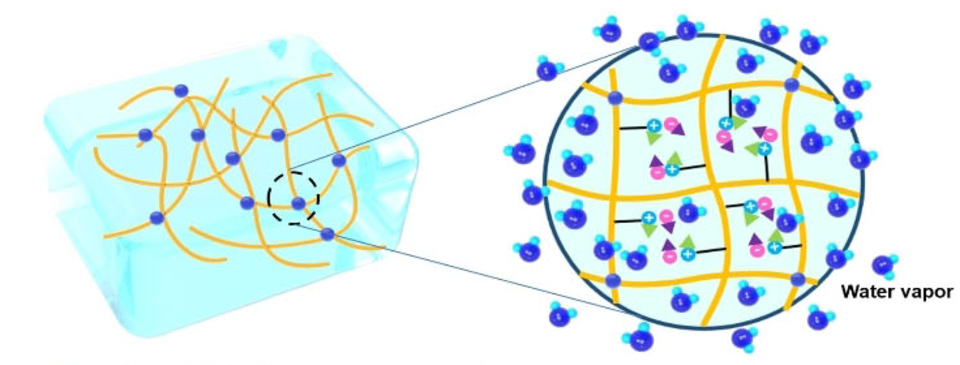
To avoid liquefication of the hygroscopic salt, it can be confined in composite structures. Guihua Yu, University of Texas at Austin, USA, and colleagues have incorporated lithium chloride in the matrix of a zwitterionic polymer to make a hygroscopic hydrogel [10] (see Fig. 5). The salt ions cannot escape from the hydrogel when water molecules enter because they participate in the zwitterionic polymeric structure. The polymer used is poly [2-(methacryloyloxy)ethyl]dimethyl-(3-sulfopropyl)ammonium hydroxide (PDMAPS).
 |
|
Figure 5. Hygroscopic hydrogel (schematically pictured) [10]. |
The salt helps capture water vapor and simultaneously improves the solubility and swelling properties of the gel. A heater then extracts the bound water as vapor, which is collected as liquid water at ambient air temperature. One kilogram of the hydrogel can take up roughly two-thirds of its own weight in water. It will be possible to set this up in a cyclical system in order to capture water from the air 24/7, say the researchers.
In May 2022, the Yu team presented a superhygroscopic polymer film composed of thermoresponsive hydroxypropyl cellulose and Konjac glucomannan (a plant-based polysaccharide) and lithium chloride [11]. The team says the film can be used in an AWH device that operates at 14–24 cycles per day in arid environments, giving a water yield of 6 to 13 liters per kilogram of material. The cycles consist of exposing the film to air for 60 to 100 minutes for moisture uptake and heating it for 30 minutes to release the bound water and collect it on a condenser.
Another type of hygroscopic hydrogel was presented by Paul A. Kallenberger and Michael Fröba, University of Hamburg, Germany. They produced an AWH by mixing sodium alginate and calcium chloride, which reacted to form a stable composite structure [12]. The dried, porous beads could take up 100 % of their weight in water. The researchers suggest an entirely passive application: Ambient air is led through the material at night for absorption, the packed bed is heated by the sun to achieve re-vaporation of the bound water molecules, and the liquid water collected at ambient temperatures.
MOFs – The Designer Approach
Porous materials are efficient desiccants, too. However, they have to be designed so that water molecules do not enter the tunnels only to become trapped there; their exit must be as smooth as possible, too. Metal-organic frameworks (MOFs) collect water molecules at their open metal sites in the form of layers or clusters. Desorption can be influenced by customizing the hydrophilicity of the functional groups [13].
One of the first MOF-based atmospheric water harvesters (AWHs) was Zr-based MOF-801, developed by the team of Omar Yaghi, University of California, Berkeley, USA. It collected and released 0.1 L of water per kilogram MOF per day at 10 % relative humidity [14].
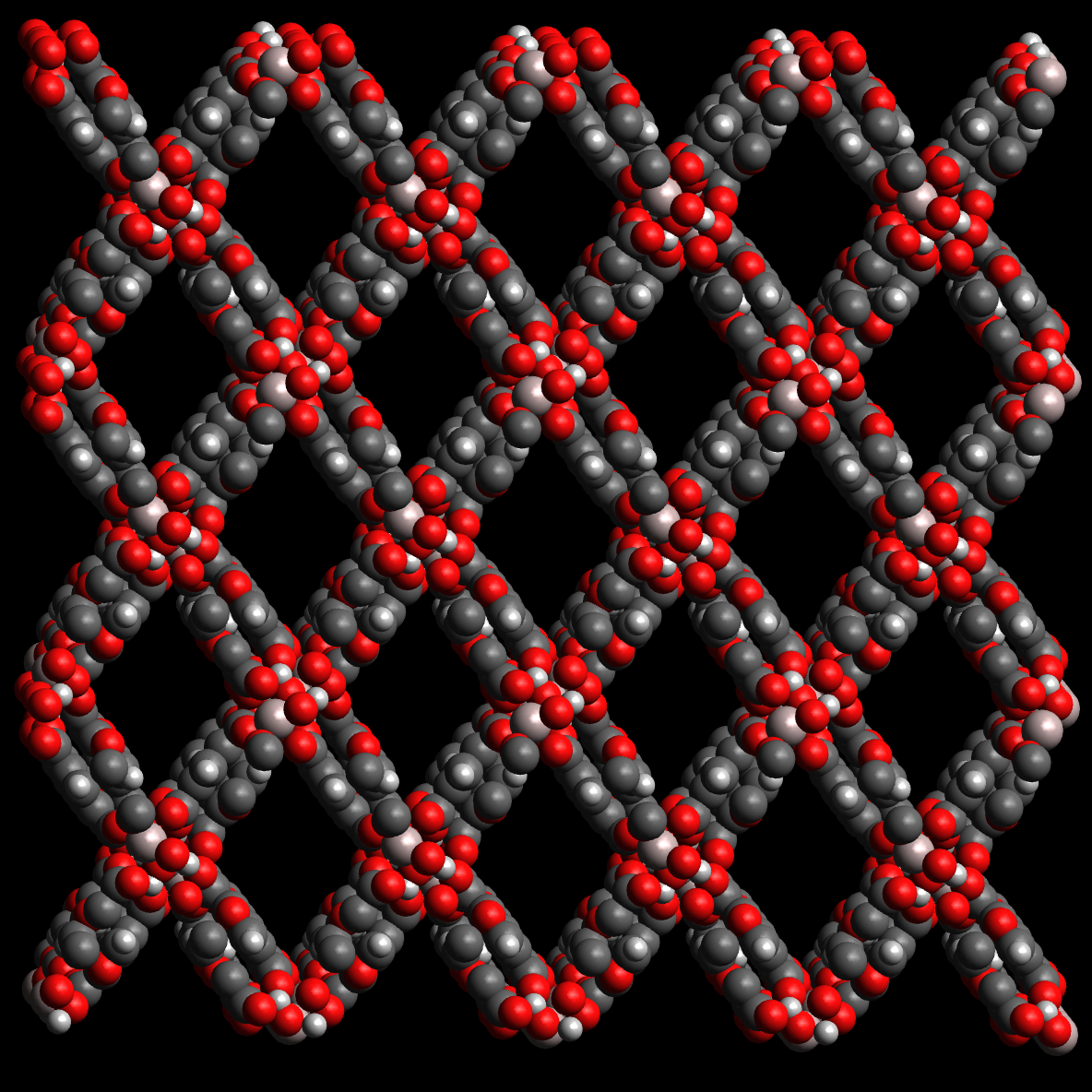
A more modern successor is MOF-303, Al(OH)(1H-pyrazole-3,5-dicarboxylate). The rod-like MOF-303 crystal structure has long channels to facilitate the uptake and release of water molecules. It delivered 0.7–1 L water per kilogram of MOF per day when tested in the Mojave desert in California where the relative humidity is below 7 % [15]. A solar panel provided the energy for desorption and water collection.
 |
|
Figure 6. The rod-like MOF-303 crystal structure has long channels to facilitate the uptake and release of water molecules. |
Yaghi founded Water Harvesting Inc., USA, to commercialize MOF-based AWH systems. The company has reported prototypes of table-top devices that can deliver up to 40 L water per kilogram of MOF per day, achieving 200 cycles of sorption and desorption accompanied by water collection, using energy input provided by solar panels [16].
Just Drinking Water – Or More?
To date, technologies for harvesting atmospheric water have been designed for drinking water purposes only. They could help replace bottled or tap water in areas where air humidity is moderate to high, and where an energy source is available. In their current form, the prospects of even larger scale applications, for example, for agriculture in the desert, are rather dim.
The ongoing challenge is to find more efficient materials and to lower the required energy input. Researchers are now concentrating on materials that have their own integrated energy source. If successful, the future could hold windshields with water trickling down them, without visible air humidity having to be present.
References
[1] C. R. Tracy et al., Condensation onto the skin as a means for water gain by tree frogs in tropical Australia, Am. Nat. 2011, 178, 553–558. https://doi.org/10.1086/661908
[2] K. Wan et al., Bio-inspired Fog Harvesting Materials: Basic Research and Bionic Potential Applications, J. Bionic. Eng. 2021, 18, 501–533. https://doi.org/10.1007/s42235-021-0040-0
[3] C. Liu et al., Effective directional self-gathering of drops on spine of cactus with splayed capillary arrays, Sci. Rep. 2015, 5, 17757. https://doi.org/10.1038/srep17757
[4] A. R. Parker, C. R. Lawrence, Water capture by a desert beetle, Nature 2001, 414, 33–34. https://doi.org/10.1038/35102108
[5] Watergen Inc.,Watergen Mobile Box, www.watergen.com. (accessed July 20, 2022)
[6] F. A. Brigano, E. A. Kapustin, Applications & Evolution of Atmospheric Water Generation Technologies, www.wqpmag.com. (accessed July 20, 2022)
[7] Fraunhofer-Institut für Grenzflächen- und Bioverfahrenstechnik IGB, WaLu – Producing Drinking Water from Air Humidity, www.igb.fraunhofer.de. (accessed July 20, 2022)
[8] Fraunhofer-Institut für Grenzflächen- und Bioverfahrenstechnik IGB, Systems Solutions for Water Supply, Water Treatment and Wastewater Purification, www.igb.fraunhofer.de. (accessed July 20, 2022)
[9] Fraunhofer-Institut für Grenzflächen- und Bioverfahrenstechnik IGB, Jahresbericht 2017|18, www.igb.fraunhofer.de. (accessed July 20, 2022)
[10] C. Lei et al., Polyzwitterionic Hydrogels for Efficient Atmospheric Water Harvesting, Angew. Chem. Int. Ed. 2022, 61, e202200271. https://doi.org/10.1002/anie.202200271
[11] Youhong Guo, Weixin Guan, Chuxin Lei, Hengyi Lu, Wen Shi, Guihua Yu, Scalable super hygroscopic polymer films for sustainable moisture harvesting in arid Environments, Nat. Commun. 2022, 13, 2761, https://doi.org/10.1038/s41467-022-30505-2
[12] P. A. Kallenberger, M. Fröba, Water harvesting from air with a hygroscopic salt in a hydrogel–derived matrix, Commun. Chem. 2018, 1, 28. https://doi.org/10.1038/s42004-018-0028-9
[13] Water Harvesting Inc., Water Harvesting at the Smithsonian, wahainc.com. (accessed July 20, 2022)
[14] H. Kim et al., Adsorption-based atmospheric water harvesting device for arid climates, Nat. Commun. 2018, 9, 1191. https://doi.org/10.1038/s41467-018-03162-7
[15] F. Fathieh et al., Practical water production from desert air, Sci. Adv. 2018, 4, eaat319. https://doi.org/10.1126/sciadv.aat3198
[16] C.-H. Liu et al., Reticular Chemistry and Harvesting Water from Desert Air, AsiaChem Mag. 2020, 18–25. https://doi.org/10.51167/acm00007
Also of Interest
- Self-Optimizing Device for Atmospheric Water Harvesting,
Roswitha Harrer,
ChemistryViews 2022.
https://doi.org/10.1002/chemv.202200077
Adaptive MOF-based device improves yields and power consumption







I would like to build a small unit for home use because of all the ecoli in our water
Am a student at the University of Malawi, am studying chemistry. I would like to know more about atmospheric water generators so that I can build a small unit for the benefit of my community and the country due to climate change depending on rain fed agriculture has proven to be ineffective.