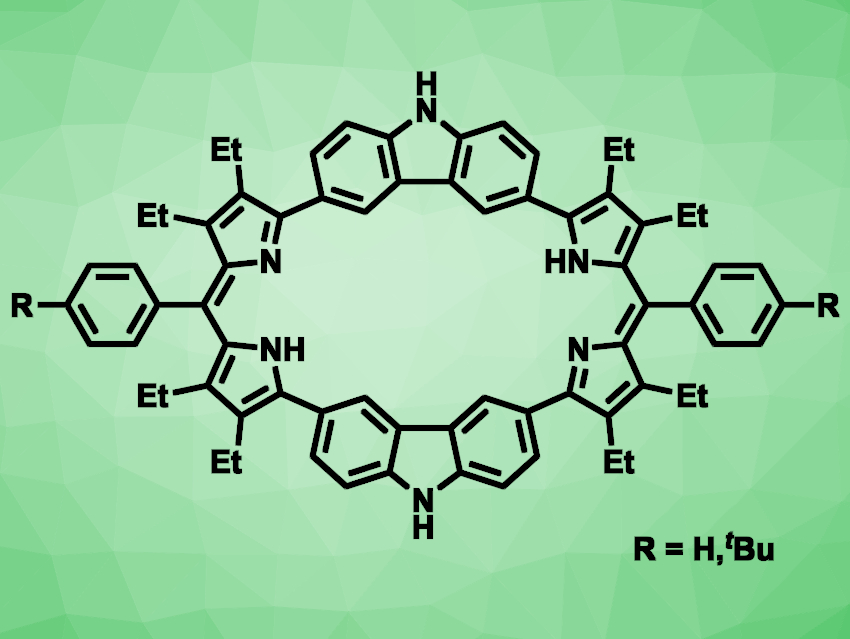
Porphyrins are macrocyclic organic compounds, containing four pyrrole units connected via =CH− bridges. Expanded porphyrins—larger analogues of these compounds—can be interesting, e.g., for their electronic and optical properties and their potential applications as ligands. Carbazole consists of two benzene rings fused to either side of a five-membered ring containing a nitrogen atom. It can be considered an analogue of pyrrole with a larger π-conjugated framework.
Jonathan L. Sessler, The University of Texas at Austin, USA, Huakang Yu, South China University of Technology, Guangzhou, and China−Singapore International Joint Research Institute, Guangzhou, China, Chuanhu Lei, Shanghai University, China, and colleagues have synthesized two carbazole-containing expanded porphyrins (pictured), called octaphyrins, as well as the bis-BF2 complex of the tBu-substituted octaphyrin.
The team started from N-Boc-2-pyrroleboronic acid, which was reacted with 3,6-dibromo-9H-carbazole in a Suzuki–Miyaura cross-coupling reaction. The resulting intermediate was decarboxylated, followed by a condensation with benzaldehyde or a benzaldehyde derivative and an oxidation with 2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ) in air to give the desired compounds.
The octaphyrin with R = H was obtained in a yield of 12 %, and the tBu-substituted derivative in a yield of 15 %. The latter was more soluble and was reacted with BF3•EtO2 in the presence of triethylamine to obtain the corresponding bis-BF2 complex. In this complex, two BF2 units are bound to the respective nitrogen atoms of the octaphyrin’s two dipyrromethane subunits. The researchers used this compound in a organic microlaser. According to the team, it is the first example of a BODIPY-type expanded porphyrin to show solid-state lasing.
- 3,6-Carbazoylene Octaphyrin (1.0.0.0.1.0.0.0) and Its Bis-BF2 Complex,
Hao Chen, Xusheng Shi, Yipeng Lun, Yan Xu, Tian Lu, Zhiming Duan, Min Shao, Jonathan L. Sessler, Huakang Yu, Chuanhu Lei,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.2c01240