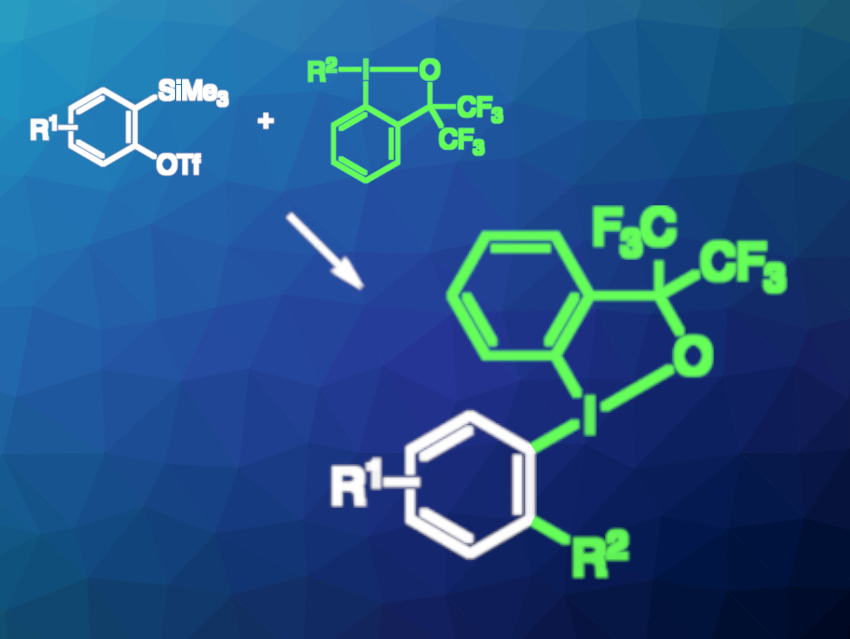
Organoiodine(III) compounds usually can be employed as carbon electrophiles. However, Naohiko Yoshikai, Tohoku University, Sendai, Japan, and colleagues have found that tailored organoiodine(III) compounds can behave more like organometallic nucleophiles in reactions with arynes, which have an electrophilic C–C triple bond. In this type of reaction (pictured), the organoiodine(III) compounds undergo an addition across the C−C triple bond. The iodine center remains trivalent after the transformation.
The team used ortho-(trimethylsilyl)phenyl triflates as aryne precursors and organobenziodoxoles with a bis(trifluoromethyl)benzyl alcohol substructure as the organoiodine(III) reagents (pictured in green). The reactions were performed in the presence of CsF, using acetonitrile as the solvent, at room temperature. This approach allowed for the introduction of alkynyl groups (R2) in moderate to high yields. Alkenyl groups could also be transferred in moderate to good yields in most cases. Electron-rich (hetero)-aryl reagents were also suitable for the reaction.
The iodine(III) group in the products is useful for further transformations, e.g., transition metal-catalyzed cross-coupling reactions to form new C–C bonds, iodine-(III)-to-iodine(I) conversions to give aryl iodides, or base-mediated aryne generation followed by cycloadditions to trap the aryne. Overall, the work provides a method for the carbohalogenation of arynes, giving products that are well-suited to further functionalization and the construction of complex structures.
- Carboiodanation of Arynes: Organoiodine(III) Compounds as Nucleophilic Organometalloids,
Chisaki Arakawa, Kazuya Kanemoto, Katsuya Nakai, Chen Wang, Shunya Morohashi, Eunsang Kwon, Shingo Ito, Naohiko Yoshikai,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.3c11524