Methods for the synthesis of value-added compounds from simple, readily available chemical feedstocks such as ethylene or carbon monoxide are useful tools in organic synthesis. Ethylene and CO can be used together, for example, in hydrocarbonylations, dicarbonylations, carbonylative difunctionalizations, etc.
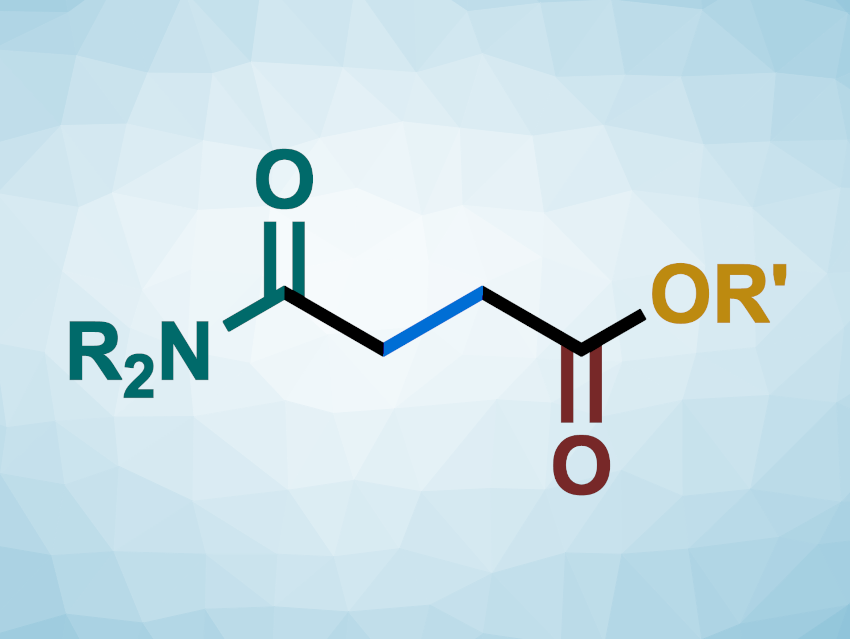
Xiao-Feng Wu, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, and Leibniz-Institut für Katalyse e.V., Rostock, Germany, and colleagues have developed a method for the carbonylative difunctionalization of ethylene for the synthesis of 4-oxobutanoates (general structure pictured), based on a ligand-free, cobalt-catalyzed four-component radical reaction. In addition to ethylene and CO, the team used formamides and alcohols to introduce both an amide and an ester group into the product. The team used CoCl2 as the catalyst and di-tert-butyl peroxide (DTBP) as a radical initiator. The reactions were performed in acetonitrile at 130 °C.
Using this approach, they obtained the desired products in mostly moderate yields. The substrate scope includes primary, secondary, and cyclic alcohols, as well as phenols with electron-donating groups or halogen atom substituents. Different formamides could be used.
Overall, the developed method provides an efficient way to install both amide and ester units in a product in one step and in an atom-economical manner. The reagents are readily available and the catalyst is based on an Earth-abundant metal.
- Cobalt-Catalyzed Carbonylative Synthesis of 4-Oxobutanoates from Formamide and Ethylene,
Le-Cheng Wang, Nai-Xian Sun, Chang-Sheng Wang, Kai Guo, Xiao-Feng Wu,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c02973