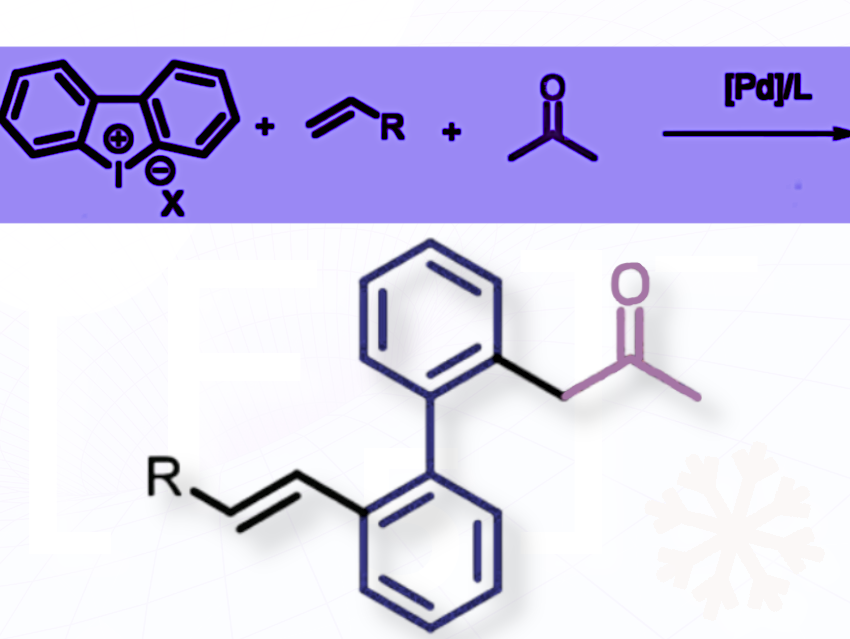
Malleswara Rao Kuram and colleagues at the CSIR−Central Drug Research Institute, Lucknow, Uttar Pradesh, India, and Academy of Scientific and Innovative Research (AcSIR), Ghaziabad, India, have developed a palladium-catalyzed reaction that uses acetone, alkenes, and cyclic diaryliodonium salts together to achieve a long-sought goal: selective mono-α-arylation of acetone, done at room temperature with a simple ligand (pictured above).
The reaction works through a cascade step: alkenylation first stabilizes the palladium complex, which then allows the acetone to be arylated in a controlled way. Acetone usually overreacts in these transformations, but here, thanks to the cascade, it behaves in just the right way.
This method provides an efficient, mild, and scalable route to bifunctionalized molecules that are valuable in drug discovery and materials chemistry, without relying on expensive custom ligands.
- Ligand-Enabled Room-Temperature Three-Component Strategy for Mono-α-arylation of Acetone with Cyclic Diaryliodonium Salts and Alkenes
Naveen Kumar Maurya, Chandra Shekhar Maurya, Malleswara Rao Kuram
Org. Lett. 2025
https://doi.org/10.1021/acs.orglett.5c02004