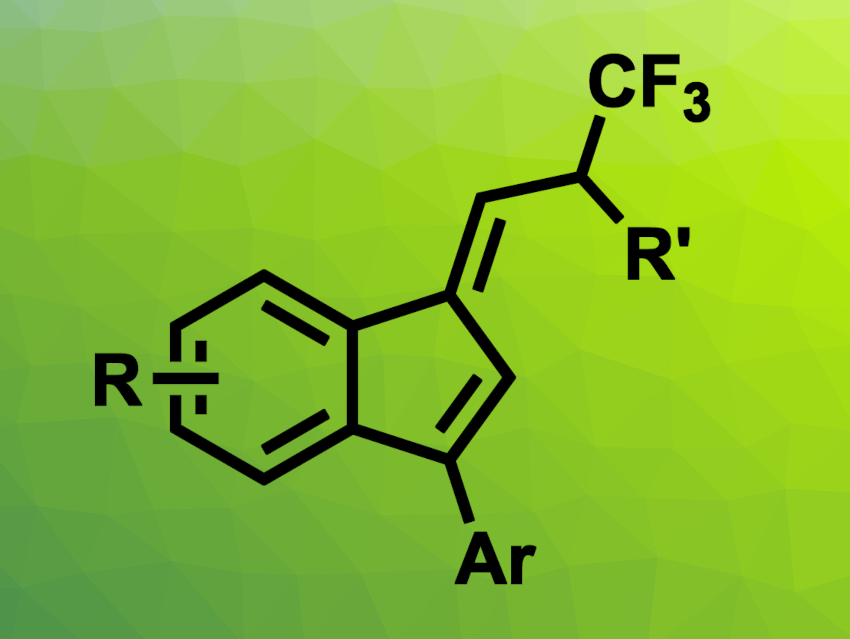
Benzofulvenes are composed of a benzene ring fused to a fulvene, an unsaturated five-membered ring with an exocyclic double bond. They can be useful, e.g., in materials science, polymer chemistry, and drug development. New methods for the synthesis of functionalized benzofulvenes are, thus, useful. In particular, approaches that provide good stereoselectivity for the E/Z configuration of the exocyclic double bond would be interesting.
Nagender Punna, CSIR-Indian Institute of Chemical Technology, Hyderabad, and Academy of Scientific & Innovative Research (AcSIR), Ghaziabad, India, and colleagues have developed a palladium-catalyzed cascade reaction that gives CF3-functionalized benzofulvenes (general product structure pictured). The team reacted CF3-functionalized 4-(2-alkynylphenyl)-allylcarbonates with aryl boronic acids in the presence of Pd(PPh3)4 as a catalyst and AcOH, using 1,4-dioxane as the solvent. The reactions were performed at 90 °C.
Under these conditions, the desired benzofulvene derivatives were obtained in good yields and with high E stereoselectivity. The cascade process involves an arylation, followed by a Trost–Oppolzer type alder ene reaction that closes the new ring, and a double-bond isomerization. The first two of these steps are promoted by the same Pd catalyst. Overall, the reaction provides access to a wide range of CF3-functionalized benzofulvenes.
- Access to CF3-benzofulvenes via palladium-catalyzed cascade arylation/Trost-Oppolzer cyclization/double-bond isomerization,
Rami Sateesh, Jaggaraju Prudhviraj, Chiliveru Priyanka, Nagender Punna,
Chem. Commun. 2024.
https://doi.org/10.1039/D3CC06082A