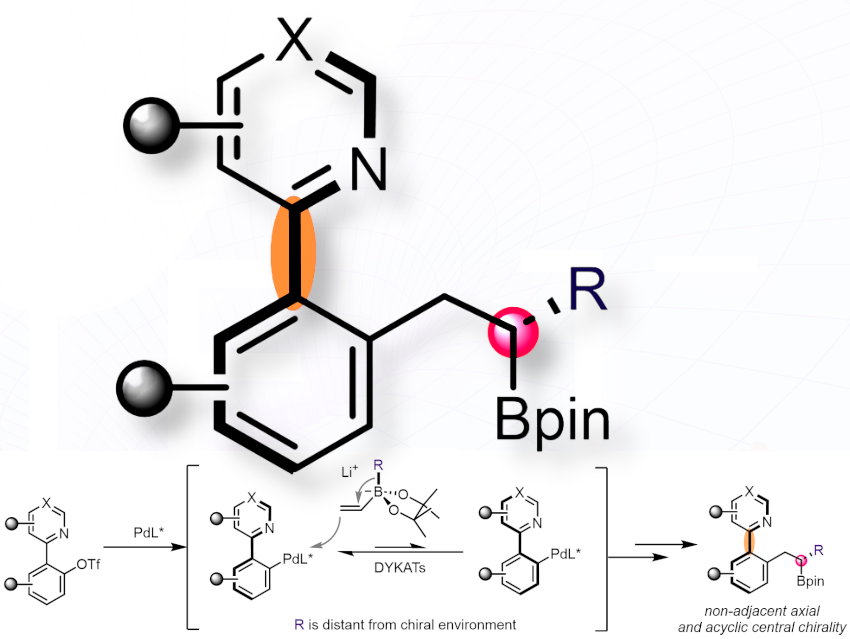
While asymmetric catalysis has seen tremendous advances—particularly in constructing single and adjacent stereocenters—methods for creating non-vicinal stereoelements have lagged, despite their prevalence in drugs and bioactive molecules.
De-Wei Gao and colleagues, ShanghaiTech University, China,addresses one of the most challenging issues in asymmetric synthesis: the simultaneous construction of non-adjacent axial and flexible, acyclic central chirality using a single palladium catalyst. The team conducted extensive ligand screening, followed by rational structural modification and optimization, ultimately identifying a bulky P,N-phosphinooxazoline ligand derived from L-serine. This ligand enables the direct asymmetric synthesis of atropisomers with non-vicinal axial chirality and flexible, acyclic central chirality.
This reaction demonstrates a broad substrate scope, including natural product derivatives, highlighting its robustness and practical utility. Mechanistic studies—covering directing group effects, intermediate characterization, kinetic analysis, and competition experiments—support a Pd(II) oxidative addition pathway, with the 1,2-metallate shift as the rate-determining step. Subsequent derivatization of the products further demonstrates the method’s effectiveness as a toolkit for synthesizing structurally complex molecules.
This approach enables the efficient construction of complex, three-dimensional molecules important for pharmaceuticals, catalysts, and materials, overcoming challenges of controlling chirality in flexible, nonadjacent positions.
- Asymmetric synthesis of chiral boronic esters featuring nonadjacent axial and flexible, acyclic central chirality,
Xi-Zhang Zou, Yu-Wen Sun, De-Wei Gao,
Science Advances 2025, 11, eadz8755.
https://doi.org/10.1126/sciadv.adz8755