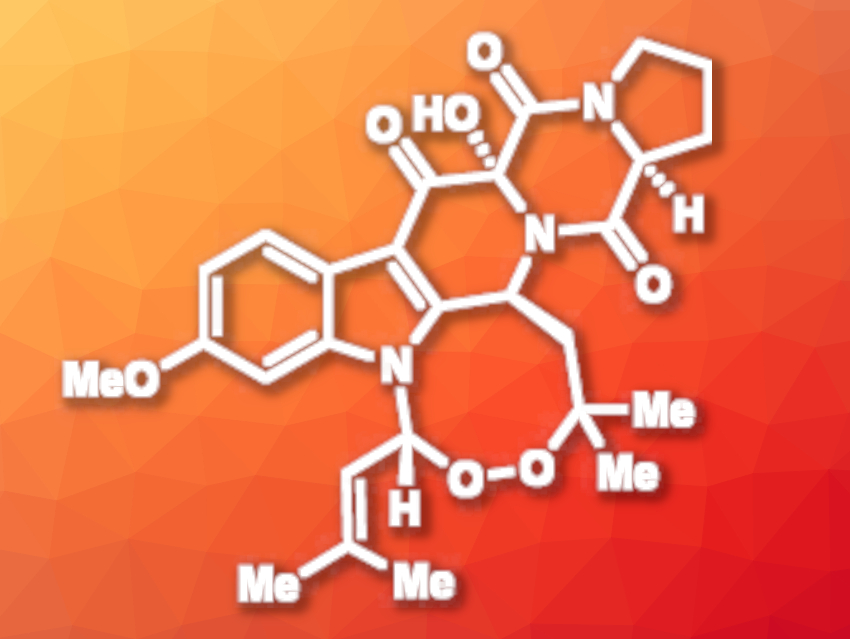
Natural products that feature organic peroxides can have useful biological activities. 13-oxoverruculogen (pictured), for example, is a fungal alkaloid that has shown promising cytotoxicity against different cancer cell lines.
Chi P. Ting, Brandeis University, Waltham, MA, USA, and colleagues have performed a concise, chemoenzymatic synthesis of 13-oxoverruculogen. The synthetic strategy involves a late-stage C–H enzymatic peroxidation to introduce the peroxo group. The team started from commercially available racemic N-carboxybenzyl-α-phosphonoglycine trimethyl ester, which was converted to a carboxylic acid and then coupled to L-proline benzyl ester to give an amide. A hydrogenolysis step, a cyclization, a Horner–Wadsworth–Emmons reaction, and an alkylation with (2-bromoethylidene)cyclopropane then gave a cyclopropane-functionalzed intermediate. A Rh-catalyzed isomerization of alkylidene cyclopropanes was then used to form the pentacyclic core of the desired product.
Once the core of 13-oxoverruculogen had been synthesized, the team performed a dihydroxylation and then used an enzymatic process for the peroxidation. The researchers employed the enzyme verruculogen synthase (FtmOx1), which gave 13-epi-verruculogen in 62 % isolated yield under optimized conditions. According to the researchers, this is the first time that FtmOx1 has been shown to convert substrates other than its native substrate to endoperoxides. The product was oxidized to give the desired (+)-13-oxoverruculogen in ten steps overall. The work shows that enzyme-catalyzed reactions could be useful for the formation of complex peroxides.
- Chemoenzymatic Synthesis of 13-Oxoverruculogen,
Jun Yang, Brandon Singh, Gabriel Cohen, Chi P. Ting,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c07078