Chirality and luminescence can both be affected by aggregation. When molecules aggregate in solvents, new photophysical behaviors can emerge.
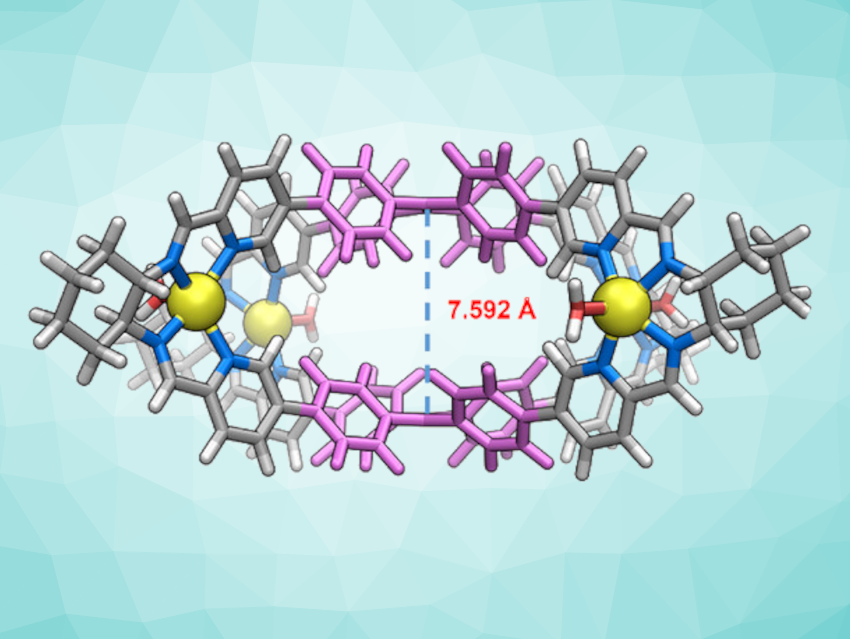
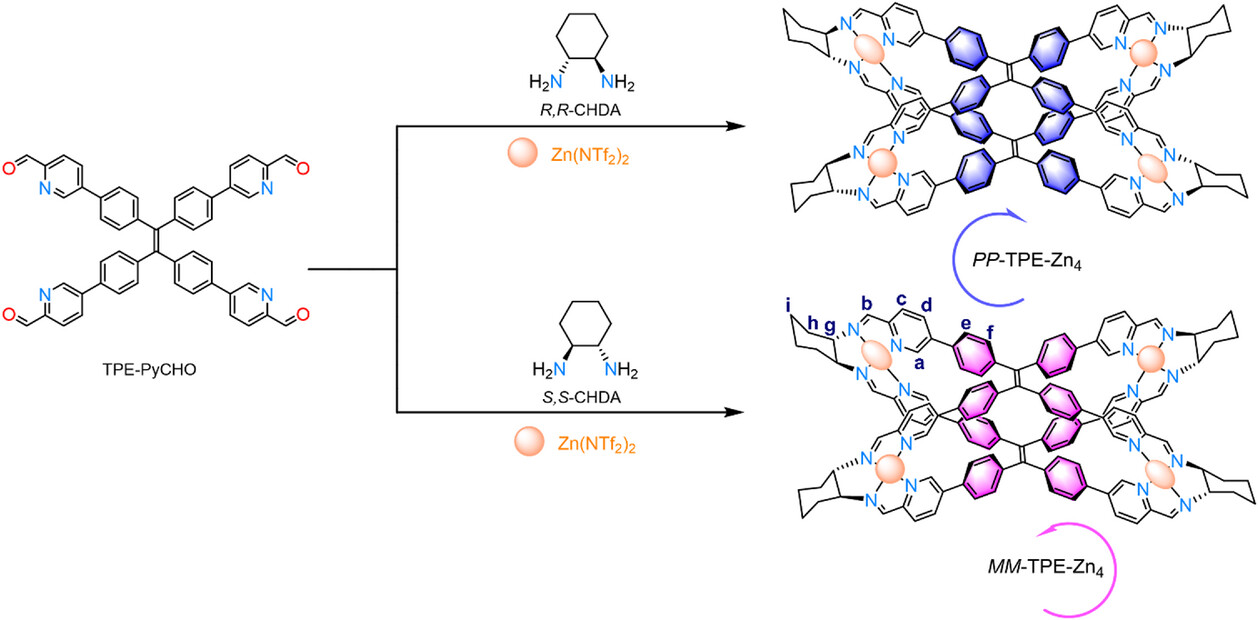
Xiao-Lan Hu, Xiao-Ping Zhou, Jinan University, Guangzhou, China, and colleagues have synthesized a pair of metalated tetraphenylethylene (TPE)-based enantiomers of an organic cage (PP/MM-TPE-Zn4, pictured below). These cages are synthesized via a self-assembly process using a luminescent tetraphenylethylene (TPE) derivative (TPE-PyCHO), chiral (R,R)/(S,S)-diaminocyclohexane (CHDA) building blocks, and zinc salts.
The cages exhibit concentration-dependent chiral behaviors. The team obtained circular dichroism (CD) spectra of PP/MM-TPE-Zn4 in dimethyl sulfoxide (DMSO) solutions at different concentrations. They found that the CD signals of the cages change when the concentration is varied, and propose that this behavior is related to the aggregation of PP/MM-TPE-Zn4 and that supramolecular chirality is generated after aggregation.
The photoluminescence (PL) properties of the cages also change at different concentrations in a DMSO solution. Interestingly, the cages show aggregation-caused quench (ACQ) behavior with increasing concentrations, but also aggregation-induced emission (AIE) when a poor solvent is added. The work could provide a strategy for designing new materials with a variety of photophysical functions by aggregating molecules.
- Homochiral metalated tetraphenylethylene‐based organic cages: Unusual chiral and luminescent behavior depending on thermodynamic and kinetic aggregation,
Hao‐Jie Zhang, Ya‐Liang Lai, Hu Yang, Xian‐Chao Zhou, Zi‐Jun Yuan, Li Deng, Xiao‐Lan Hu, Xue Li, Xiao‐Ping Zhou, Dan Li,
Aggregate 2024.
https://doi.org/10.1002/agt2.598