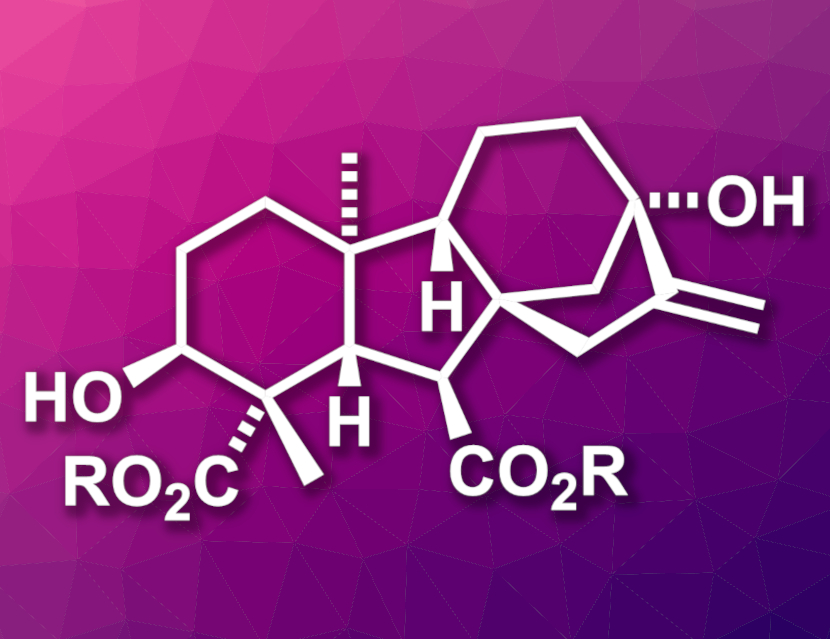
Gibberellins (GAs) are important plant hormones. They are tetracyclic diterpene acids with either 19 or 20 carbon atoms. GAs are used, e.g., in agriculture. Gibberellin derivatives could also serve as lead compounds for drug development. However, some gibberellins are in very limited supply in Nature. This includes GA18 (corresponding methyl ester pictured, R = Me), a C20 gibberellin. GA18 was isolated from immature seeds of yellow lupin in extremely low yield. An efficient synthesis of the compound would, thus, be useful.
Mingji Dai, Emory University, Atlanta, GA, USA, Purdue University, West Lafayette, IN, USA, and colleagues have developed a concise synthesis of (−)-GA18 methyl ester from the cheap diterpenoid andrographolide. The team first converted andrographolide to a triene and protected its remaining hydroxyl groups. An oxidative cleavage of a double bond using KMnO4 then gave an aldehyde intermediate. This was followed by a Wittig one-carbon homologation and an ene reaction to close a six-membered ring.
The team then used an ozonolysis and an intramolecular aldol condensation to construct the target’s trans-hydrindane. A photochemical [2+2] cycloaddition with allene and a single-electron-transfer (SET)-promoted skeletal rearrangement were then used to create the methylenebicyclo[3.2.1]octanol unit. Further deprotection, oxidation, esterification, and reduction steps then gave the desired (−)-GA18 methyl ester.
- Concise Synthesis of (−)-GA18 Methyl Ester,
Lei Li, Weida Liang, Mario E. Rivera, Ye-Cheng Wang, Mingji Dai,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.2c12470