Tonya Vitova, Stefanie Dehnen, and Wim Klopper from Karlsruhe Institute of Technology (KIT), Germany, discuss their recent study revealing covalent La–Bi interactions within an electron-rich In/Bi cluster, challenging traditional views of lanthanide bonding.
What did you do?
By a combination of advanced X-ray spectroscopic experimental tools and highly sophisticated quantum chemical calculations, we investigated a compound based on a very uncommon molecule, namely an ‘intermetalloid cluster’ comprising exclusively metal atoms in the elemental combination and composition La:In:Bi = 2:4:24.
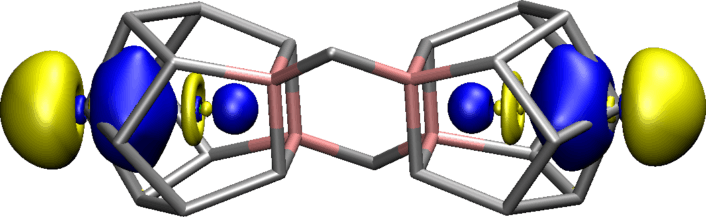
In the molecule we investigated here (pictured above), each of the lanthanum (La) atoms adopts an ‘interstitial’ position, being situated inside a polyhedral cluster shell comprising two indium (In) and 11 bismuth (Bi) atoms. Two further Bi atoms bridge two of such units, so that the complete cluster molecule, [(La@In₂Bi₁₁)₂Bi₂]⁶⁻, resembles a ‘supermolecule’ of two connected ‘superatoms’.
Intermetalloid clusters exhibit very uncommon bonding properties, which we intended to explore in detail in our study. We were particularly interested in how the La atoms interact with the surrounding In/Bi cluster shell and unraveled the situation that allowed for new insights into intermetallic bonding.
What interests you about this?
Our goals are (a) to synthesize new lanthanide-based multimetallic clusters with unusual bonding properties and (b) to understand the relationship between the bonding situation and the clusters’ chemical and physical behavior.
We are also developing high-energy-resolution X-ray spectroscopic tools and quantum chemical methods designed to provide in-depth insights into lanthanide–ligand bonding. These tools are intended to be used to support the development of synthetic strategies that aim at tuning molecular characteristics at the boundary between covalent and metallic bonding, thereby advancing the chemical and physical properties of novel multinary cluster compounds.
What is new and cool about your work?
It is widely accepted that lanthanides primarily form ionic bonds in molecular compounds. However, we demonstrate that this is not the case for an unconventional multimetallic cluster and its precursor molecule. Surprisingly, the most covalent molecular orbital in the cluster resembles the bonding in XeF₂. Molecules composed exclusively of metal atoms are generally rare compared to organic or organometallic species, so the knowledge gained from each new example adds massively to our understanding of chemical bonding.
The combined multi-element X-ray spectroscopic and quantum chemical approach used herein has never before been applied to intermetalloid clusters, in particular not to clusters incorporating single lanthanide atoms.
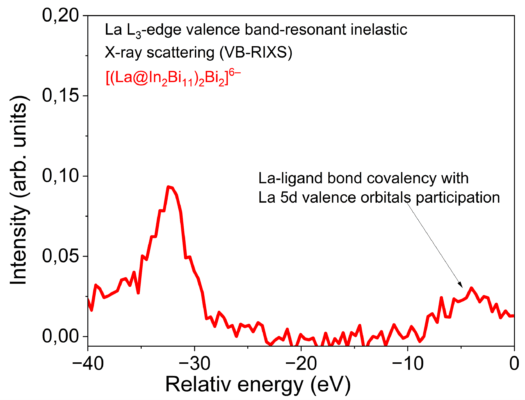
What are your key findings?
We revealed that the encapsulation of a single lanthanide ion in an In/Bi cage offers a non-standard bonding situation leading to a clearly detectable covalent La(5d)–Bi(6p) interaction. This is observed in the highly electron-rich environment of the ternary cluster [(La@In₂Bi₁₁)₂Bi₂]⁶⁻. The most prominent covalent molecular orbital is reminiscent of the bonding interaction in XeF₂.
Our results indicate that lanthanide atoms are capable of forming bonds with significant covalent character when placed in a suitable ligand environment. Advanced X-ray spectroscopic experimental tools focusing on the different metals La, In, and Bi within the intermetalloid cluster and quantum chemical calculations were applied for comparative studies of the bonding situation.
What is the longer-term vision for your research?
Given their high reactivity towards small molecules, and the high flexibility in their composition – including rather benign metal atoms – intermetalloid clusters show a high potential as atomically precise metal nanocatalysts. The design and optimization of catalytic properties aims at the discovery of a sweet spot in reactivity and selectivity, which can be reached by a subtle adjustment of composition and structures. This, in turn, is facilitated and accelerated by a comprehensive knowledge of the bonding in the clusters, which is instrumental to reach the goal, as the bonding properties of the catalysts represent the basis of their chemical behavior.
Another goal is the use of ternary clusters for the formation of metal nanoparticles with tailor-made compositions, serving either as catalysts or as precursors for new multinary alloys or nanostructured semiconductors.
What part of your work was the most challenging?
The X-ray experiments were challenging due to the cluster’s high sensitivity to air, moisture, and X-ray exposure. However, thanks to the close teamwork between the synthetic chemists and the X-ray spectroscopy teams, and the ability to perform the experiments at the on-campus KIT Light Source synchrotron – located next to the chemical laboratories and offering exceptional experimental flexibility – the experiments were successful.
Furthermore, the quantum chemical calculations had to be performed at the edge of what is technically feasible today.
Anything else you would like to add?
The work has been carried out in the framework of the DFG-funded Collaborative Research Center (CRC) SFB 1573. We would like to emphasize that we are very grateful to the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) for offering scientists in our country the opportunity to pursue their individual, creative, and also curiosity-driven research interests, which ultimately allows us to accumulate new knowledge that in the long run will help to create new materials for a better future.
Thank you very much for sharing these insights into your recent work.
The paper they talked about:
- Molecular–Metallic Binding Characteristics of the Intermetalloid f-/p-Block Cluster [(La@In₂Bi₁₁)₂Bi₂]⁶⁻,
Harry Ramanantoanina, Julia Rienmüller, Yannick Lohse, Nina Rauwolf, Max Kehry, Cedric Reitz, Emily Marie Reynolds, Tim Prüßmann, Bianca Schacherl, Viktoriia Saveleva, Ruwini Ekanayake, Jörg Göttlicher, Bastian Weinert, Wim Klopper, Stefanie Dehnen, Tonya Vitova,
Angew. Chem. Int. Ed. 2025.
https://doi.org/10.1002/anie.202512019

Tonya Vitova is a Professor of Advanced X-ray Spectroscopy in f-Element Chemistry at the Institute for Nuclear Waste Disposal, Karlsruhe Institute of Technology (KIT).

Stefanie Dehnen is a Professor of Inorganic Chemistry and Executive Director of the Institute of Nanotechnology at Karlsruhe Institute of Technology (KIT).

Wim Klopper is a Professor of Theoretical Chemistry at the Institute of Physical Chemistry and the Institute of Nanotechnology, Karlsruhe Institute of Technology (KIT).
Also of Interest
![Covalent La–Bi Bonding in the f-/p-Block Cluster [(La@In₂Bi₁₁)₂Bi₂]⁶⁻](https://www.chemistryviews.org/wp-content/uploads/2025/07/202507_LaBiInteractions.png)



