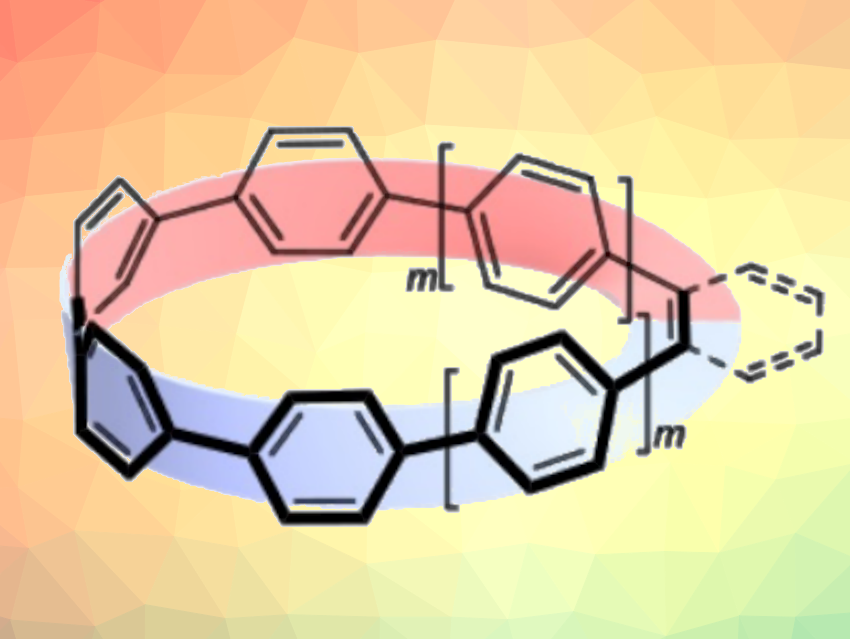
π-Conjugated molecules with a so-called Möbius topology have a circular structure in which a portion of the ring is twisted. Although such molecules are chemically and structurally interesting, general methods for their synthesis remain limited, and little is known about their physical properties and functionality.
Eiichi Kayahara, Shigeru Yamago, Kyoto University, Japan, and colleagues have synthesized a range of cyclic π-conjugated molecules with a Möbius topology. The team used a series of coupling reactions to fuse cycloparaphenylene (CPP) precursors with ethylene or ortho-phenylene units to create twisted CPPs. They synthesized mono-alkene-inserted [N]CPPs with N = 6, 8, and 10, mono-ortho-phenylene-inserted [6]CPP, and di-alkene-inserted [N]CPP with N = 4, 6, and 8. The mono-alkene and ortho-phenylene derivatives have a Möbius-type structure (pictured below on the left), while the di-alkene derivative exhibits a double twisted structure (pictured below on the right).
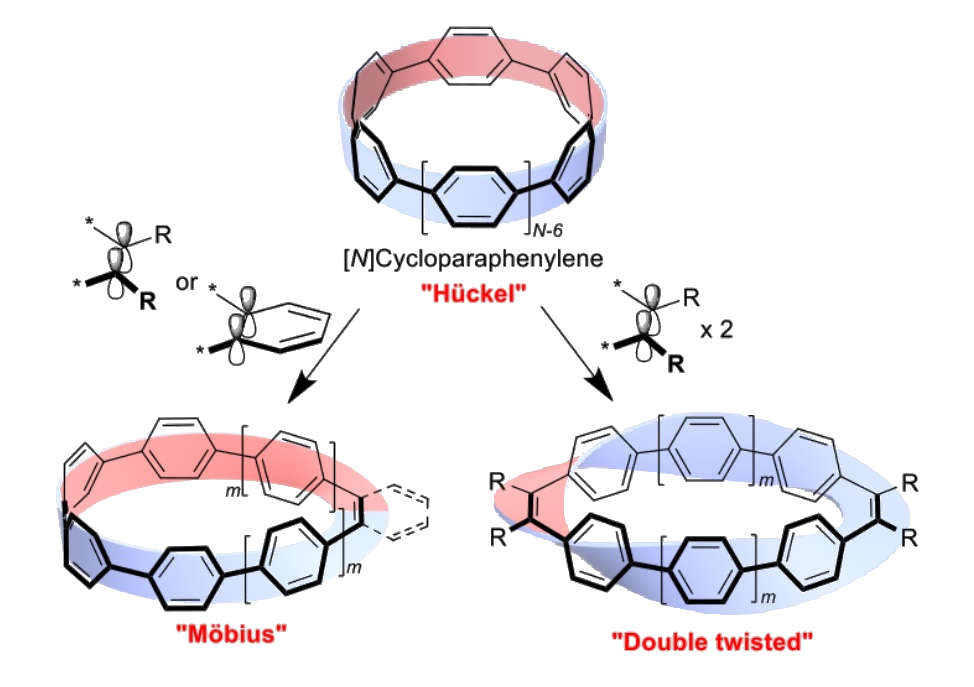
In the crystalline state, all Möbius-type products preserved their topology. While the Möbius topology was lost in solution for the parent compounds, a mono-alkene-inserted [6]CPP functionalized with eight additional pyrrole rings retained a Möbius structure even in solution, as determined by variable-temperature NMR spectroscopy. According to the researchers, this research could be useful for the creation of new cyclic π-conjugated molecules with various topologies and properties.
- Synthesis of Twisted [N]Cycloparaphenylene by Alkene Insertion,
Tomoaki Terabayashi, Eiichi Kayahara, Yichen Zhang, Yoshiyuki Mizuhata, Norihiro Tokitoh, Tohru Nishinaga, Tatsuhisa Kato, Shigeru Yamago,
Angew. Chem. Int. Ed. 2022.
https://doi.org/10.1002/anie.202214960