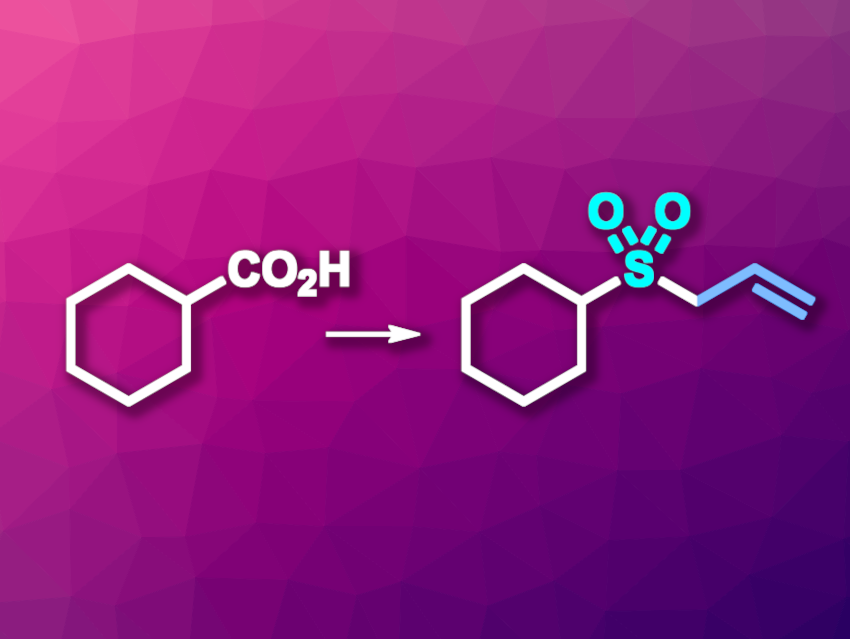
Carboxylic acids are widely available chemical feedstocks and useful precursors in organic synthesis. The development of methods to, for example, use carboxylic acid groups to introduce carbon–heteroatom bonds is interesting. Approaches that use catalysts based on Earth-abundant metals, such as iron, and/or photochemistry can be particularly useful from a sustainability perspective.
Rong Zeng, Xi’an Jiaotong University, China, and colleagues have developed a method for the photoinduced, iron-catalyzed decarboxylative sulfonylation of carboxylic acids (example reaction pictured). The reaction gives useful organic sulfones. The team reacted a variety of both acyclic and cyclic carboxylic acids with 1,4-diazabicyclo[2.2.2]octane·(SO2)2 (DABSO) and electrophilic reagents such as allyl bromide, using TBAFeCl4 (TBA = tetrabutylammonium) as the catalyst, triethylamine as a base, and dichloromethane (DCM) as the solvent. The reactions were performed under LED irradiation (390 nm) and a nitrogen atmosphere at room temperature.
Under these conditions, the desired sulfones were obtained in moderate to high yields. The reaction has a broad substrate scope and proceeds under mild conditions. The team proposes a reaction mechanism that involves a single-electron transfer (SET) from the carboxylic acid to Fe(III), generating an RCO2• radical. This radical releases CO2, and the resulting alkyl radical reacts with DABSO to form an intermediate of the type RSO2•. Another electron transfer regenerates the catalyst and produces an RSO2–-type species, which is trapped by the electrophile reaction partner to give the product.
- Decarboxylative Sulfonylation of Carboxylic Acids under Mild Photomediated Iron Catalysis,
Yuanqi Dong, Ni Xiong, Zhouting Rong, Rong Zeng,
Org. Lett. 2024.
https://doi.org/10.1021/acs.orglett.4c00410