Capillary spotters, or TLC spotters, are very useful for depositing small amounts of sample onto a TLC (thin layer chromatography) plate. This prevents overloading and helps achieve an accurate and clear TLC in preparation for running a full column. TLC spotters are easy to make and are a much better alternative to trying to use a Pasteur pipette which have a wider opening and so create a larger spot on the TLC – no matter how careful you are.
TLC spotters are made from glass capillary tubes, available from most suppliers of chemicals or glassware. A capillary tube with two open ends will create two TLC spotters. A capillary melting point tube – with one end closed – will create one TLC spotter.
To Make TLC Spotters (Fig. 1)
- Heat the middle of a glass capillary tube over a blue Bunsen flame. Hold the ends carefully as they will get hot! Use heat-proof gloves or tweezers.
- When the middle is hot enough, it will become pliable. Pull the ends apart quickly and smoothly. This will draw the middle into a thin, string-like tube.
- Bring the two ends you are holding together. This will cause the thin middle section to bend and ultimately snap.
- Shorten the thin end of the spotters by snapping them to the desired length by bending the glass. A workable length is 3 cm max. Any longer and the tips will snap when you touch them to the TLC plate. The end should be as flat as possible as jagged edges can lead to overloading, unsymmetrical spots, or no spots at all on the TLC plate.
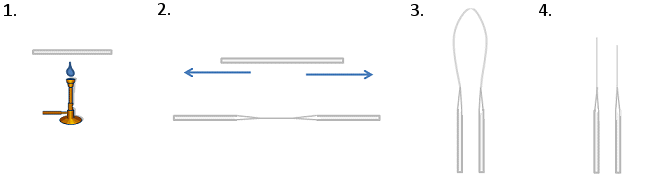
Figure 1. Production of TLC spotters.
To Use a TLC Spotter
- Place a small amount of your sample (2–5 mg) in a clean vial or beaker.
- Dissolve in suitable solvent (1–2 ml).
- Hold the narrow end of the spotter in the solution and let the solvent be drawn up into the spotter by capillary action until the thin portion of the spotter is 3/4-full.
- Touch the end to the TLC plate and allow capillary action to draw the solution onto the plate. It is better to touch the spotter to the TLC 2–3 times for short amounts of time (> 1s) than to hold it against the plate for a longer time. The resulting spot should be 1–2 mm in diameter.
- Once you have enough sample deposited on the TLC plate, touch the spotter to a piece of tissue to remove any remaining sample.
- Dispose of used spotter in a sharps container.
Reuse
TLC spotters can be reused, however, this can lead to cross contamination so should be avoided if you are in any doubt.
To clean a TLC spotter, you will need:
- A vial or small beaker with suitable solvent.
Dichloromethane is often a good choice as are ethyl acetate or whichever solvent you are using for your column. - A tissue or absorbent paper.
Method:
- Touch the TLC spotter to the tissue to remove any of the previous sample that remains in the spotter.
- Hold spotter in vial or beaker with solvent. Allow solvent to be drawn up by capillary action.
- Touch spotter to tissue until empty.
- Repeat steps 2 and 3 three to five times.
With reuse, the tips of TLC spotters can get damaged or clogged with silica from the TLC plate. If this occurs, the end of the tip can be snapped off by bending. The end should be as flat as possible as jagged edges can lead to overloading and unsymmetrical spots on the TLC plate.
Article Information
https://doi.org/10.1002/chemv.201200153


