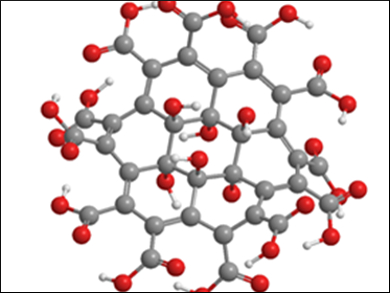
Stoichiometric graphene derivatives, such as fluorographene (C1F1)n or graphane (C1H1)n are highly sought-after members of the graphene family. With their well-defined structures, defined bandgaps, and the presence of functional groups for high-density functionalization of the carbon framework, they are interesting materials for new technologies.
Zdeněk Sofer, University of Chemistry and Technology, Prague, Czech Republic, and colleagues have developed the first synthesis of graphene acid. They have synthesized a graphene derivative with an almost stoichiometric amount of carboxylic acid functional groups (ratio of C to COOH close to 1:1) by the multiple oxidation of graphene oxide to remove oxygen functional groups.
Graphene oxide, known since the 1930s, has since been widely studied in itself. Not only is it a starting material for the production of graphene, its physical properties make it an attractive material for applications, e.g., in microelectronic devices and composite materials. Graphene oxide is itself not a well-defined derivative of graphene due to the presence of different oxygen functional groups (e.g., hydroxyl, carboxyl, and carbonyl groups) throughout its structure .
The team tested the graphene acid’s potential for applications for the sorption of toxic metals and gases. They found an extremely high sorption capacity towards various metal ions of up to 2.5 mmol/g and a high sorption capacity towards CO2. The material can also be used to assemble flexible, highly transparent membranes. The researchers suggest that further functionalization of the carboxylic acid groups should be easily possible and the material could be interesting for other applications, such as sensor technologies or energy storage.
- A New Member of the Graphene Family: Graphene Acid,
Ondřej Jankovský, Michal Nováček, Jan Luxa, David Sedmidubský, Vlastimil Fila, Martin Pumera, Zdeněk Sofer,
Chem. Eur. J. 2016.
DOI: 10.1002/chem.201603766