Nanoparticles with uniform sizes and morphologies are easy to synthesize for some classes of materials, such as metal or semiconductor nanoparticles. The growth processes of metal oxides, however, are more complex, which limits the possibilities for the controlled formation of monodisperse samples or anisotropic structures. Although nonaqueous synthesis is experimentally simple and leads to relatively homogeneous metal oxide nanocrystals, the underlying formation mechanisms are only partially understood.
Georg Garnweitner and colleagues, Technische Universität Braunschweig, Germany, have shown that the formation of TiO2 nanocrystals in a nonaqueous synthesis is even more complex than previously believed. Rather than a monomeric titanium alkoxide, as assumed until now, the synthesis is shown to involve an oligomeric intermediate complex with a defined composition.
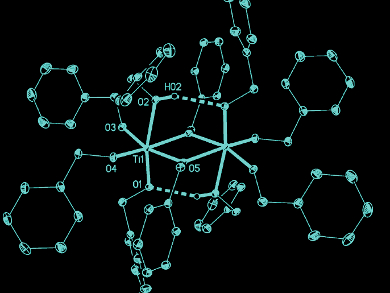
The researchers found evidence for the formation of titanium benzyloxide (pictured), a novel dimeric coordination compound, using NMR spectroscopy and single-crystal X-ray structure analysis. The team identified this dimer as a crucial compound for particle formation. It is probable that such defined coordination complexes also play a key role in the formation of other metal oxides.
- Formation of a Dimeric Precursor Intermediate during the Nonaqueous Synthesis of Titanium Dioxide Nanocrystals,
Mandy Zimmermann, Kerstin Ibrom, Peter G. Jones, Georg Garnweitner,
ChemNanoMat 2016.
DOI: 10.1002/cnma.201600264