Click Chemistry in Living Organisms
Assembling a drug from harmless components at the target location, such as a tumor, would help reduce the side effects of treatment. In the journal Angewandte Chemie, British and Malaysian scientists present a new, nontoxic catalyst made of copper nanoparticles that can be used to specifically and selectively assemble building blocks in a living system. It was shown to be possible to make an anti-tumor drug from two benign components in situ.
Chemical reactions that do not interfere with cellular processes are important in the investigation of biological functions in the native environment. The approach known as “click” chemistry makes it possible to carry out selective and highly specific reactions that are easy to carry out, even in a complex environment. In this method, molecules are “clicked” together like puzzle pieces.
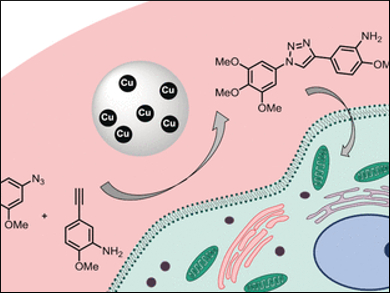
An example of such a reaction is the azide–alkyne cycloaddition reaction (developed by Meldal and Sharpless), in which an azido group (–N3) reacts specifically with an alkyne group (–C≡C–) to form a triazole, a five-membered ring with three nitrogen and two carbon atoms linked by a double bond. This reaction is often used to link biomolecules, though it has previously not been suitable for use in living systems because the copper ions required as catalyst are toxic.
Biocompatible Copper Catalyst
A new, nontoxic catalyst now makes this reaction available for new areas of application. It was developed by a team from the University of Edinburgh, UK, and the Universiti Kebangsaan Malaysia, Bangi, and consists of copper nanoparticles bound in a polymer resin.
The team successfully tested the new catalyst in biological systems ranging from cells to zebrafish. The first test used a dye that fluoresces only when two components are linked through a click reaction. In order to prove the biocompatibility of the catalyst, the researchers implanted a copper-resin sphere into the yolk sacs of zebrafish embryos that subsequently developed normally to the larval stage. After the dye precursors were added to the water, the larvae began to fluoresce, demonstrating that the catalyst is also effective as an implant.
For more tests, the researchers broke the anti-tumor drug combretastatin A-4 into two halves, equipping one with an azido group, the other with an alkyne. Using the new catalyst, the halves could be attached through the resulting triazole ring. While both of the pieces are nontoxic, the triazole product stopped the cell growth of cultivated tumor cells and initiated programmed cell death.
It may be possible to implant the copper-resin spheres into the proximity of a tumor and then use them to generate active drugs from inactive precursors on location. This would restrict the cytotoxic effects to the tumor itself.
- Copper Catalysis in Living Systems and In Situ Drug Synthesis,
Jessica Clavadetscher, Scott Hoffmann, Annamaria Lilienkampf, Logan Mackay, Rahimi M. Yusop, Sebastien A. Rider, John J. Mullins, Mark Bradley,
Angew. Chem. Int. Ed. 2016.
DOI: 10.1002/anie.201609837