Conductivity in liquid electrolytes is generally accepted to be due to the free mobility of ions in the electrolyte. Jacques-E. Moser, École Polytechnique Fédérale de Lausanne, Switzerland, and co-workers have demonstrated the existence of a mechanism for the conduction of electricity without mobile ions.
The team studied the conduction properties of the ionic liquid (IL), 1-methyl-3-propylimidazolium iodide, and the dynamical behavior of IL/I2 mixtures by using terahertz time-domain spectroscopy. At tens degrees below the liquid state, the conduction was found to be almost exactly that of its solid state, which contradicts the mobile-ion model. The Grotthuss mechanism of bond-exchange processes was determined to be a key factor in enhancing the conductivity of the melts: Iodide ions formed groups which expelled the end ions when an isolated ion approached the other end.
This has important implications from ILs used as charge-transport media in batteries or dye-sensitized solar cells.
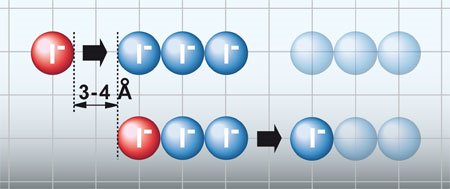
Image: (c) École Polytechnique Fédérale de Lausanne, Switzerland
- Extraordinarily Efficient Conduction in a Redox-Active Ionic Liquid
V. K. Thorsmølle, G. Rothenberger, D. Topgaard, J. C. Brauer, D.-B. Kuang et al.,
ChemPhysChem 2011, 12(1), 145–149.
DOI: 10.1002/cphc.201000819




