“Stranded” Gas Reserves
Satellite images reveal more than 7,000 natural gas flaring sites worldwide, amounting to 3.5 % of methane production going up in smoke annually [1]. The World Bank has launched an initiative to end routine flaring by 2030, calling the practice “unsustainable from a resource management and environmental perspective” [2]. Some 60 nations, companies, and organizations that support the initiative are now compelled to seek out industrially viable technologies to valorize methane.
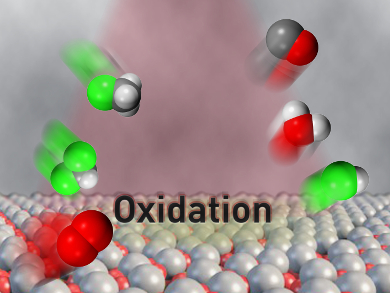
The Advanced Catalysis Engineering group at the Swiss Federal Institute of Technology (ETH) Zurich aims to capitalize on so-called “stranded” gas reserves that are earmarked for flaring because of prohibitive retrieval costs [3]. A team led by Javier Pérez-Ramírez has developed a gas-phase process to selectively convert methane into carbon monoxide, which is a versatile building block for the manufacture of chemical commodities [4]. The process couples two catalytic reactions together in a continuous-flow fixed-bed reactor over a vanadyl pyrophosphate catalyst.
In the first step, C–H activation occurs by oxychlorination using hydrogen chloride and oxygen to form chloromethanes, and in the second step, oxidative dehydrohalogenation produces carbon monoxide and regenerates the acid.
Methane: A Tough Nut to Crack
Methane valorization is challenging because of the symmetric nature of the molecule and the electronic equivalence of its four strong C–H bonds [5]. Transformation strategies usually require aggressive reaction conditions and may call for a catalyst – conditions that favor mixtures of products. Lead investigator Pérez-Ramírez explains: “functional molecules are more prone to react than methane under the reaction environment, and hence undergo further reactions leading to undesired products.”
A Balancing Act
Vanadyl pyrophosphate acts on methane indirectly by first oxidizing hydrogen chloride into chlorine – an auxiliary oxidant that reacts with methane to generate intermediate chloromethanes. This strategy allows sequential oxychlorination and dehydrohalogenation steps to proceed in the same temperature window, producing carbon monoxide selectively.
Under experimental conditions, vanadyl pyrophosphate maintains 90 % selectivity at 803 K for 100 hours with no sign of catalyst degradation. The researchers suggest that the cost of an industrial operation using the technology would be mitigated because the route is exothermic and proceeds under ambient pressure and at moderate temperatures. Pérez-Ramírez has high hopes for the technology, noting that it is “particularly attractive for the development of a modular, decentralized process, enabling the on-site valorization of methane retrieved from small natural-gas basins that are located in remote areas.”
- Selective Production of Carbon Monoxide by Methane Oxychlorination over Vanadyl Pyrophosphate,
Vladimir Paunović, Guido Zichittella, Réne Verel, Amol P. Amrute, Javier Pérez-Ramírez,
Angew. Chem. Int. Ed. 2016.
DOI: 10.1002/anie.201608165
References
- [1] Methods for Global Survey of Natural Gas Flaring from Visible Infrared Imaging Radiometer Suite Data,
Christopher Elvidge, Mikhail Zhizhin, Kimberly Baugh, Feng-Chi Hsu, Tilottama Ghosh,
Energies 2015, 9, 14.
DOI: 10.3390/en9010014 - [2] Zero Routine Flaring by 2030,
World Bank Group,
http://www.worldbank.org. (accessed October 29, 2016) - [3] Unconventional Chemistry for Unconventional Natural Gas,
E. McFarland,
Science 2012, 338, 340–342.
DOI: 10.1126/science.1226840 - [4] Catalytic conversion of methane to more useful chemicals and fuels: a challenge for the 21st century,
Jack H. Lunsford,
Catal. Today 2000, 63, 165–174.
DOI: 10.1016/S0920-5861(00)00456-9 - [5] Understanding and exploiting C–H bond activation,
Jay A. Labinger, John E. Bercaw,
Nature 2002, 417, 507–514.
DOI: 10.1038/417507a