Phyllobilins, Chlorophyll Decomposition Product
Before trees lose their leaves in the winter, they offer us a bright autumnal display of reds, oranges, and yellows. This results from the decomposition of the compound that makes leaves green: chlorophyll. Among the decomposition products are yellow phyllobilins that demonstrate unusual chemical properties. As reported by Austrian scientists in the journal Angewandte Chemie, these compounds act as four-step molecular “switches” that are triggered by light in different ways depending on the environment.
During the summer, green leaves use their chlorophyll to convert sunlight into chemical energy. Before they lose their leaves in the cold season, trees reclaim important nutrients like nitrogen and minerals. “The chlorophyll released in this process must be broken down because it has a damaging effect on the tree when it is irradiated by light while unbound,” explains Bernhard Kräutler. “Presumably, the chlorophyll decomposition products play a physiological role as well.”
Yellow Chlorophylls are Environment-responsive Photoswitches
The decomposition of chlorophyll leads to the formation of phyllobilins. Most of these are colorless, but in leaves there are also yellow ones, known as phylloxanthobilins. Researchers working with Kräutler at the Universities of Innsbruck and Graz, Austria, and Columbia University, New York, USA, have demonstrated that these compounds act as unique four-stage “switches” that react to light (photoswitches). The molecular environment determines which “switching mechanism” is used.
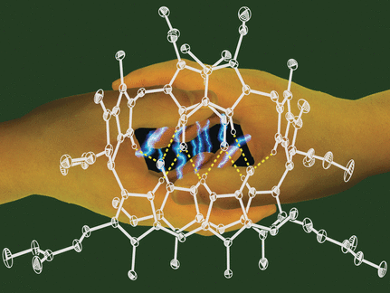
In polar media, such as the aqueous environment inside a cell, phylloxanthobilins are found as simple molecules. When irradiated with light, they switch reversibly between two forms that have slightly different spatial structures around one double bond (Z/E-isomerization). This is similar to important plant photoswitches. In nonpolar media and presumably in cellular membrane systems, the Z-isomers pair up and are held together by hydrogen bonds. Irradiation with light leads to a chemical reaction between the two paired molecules. In this cycloaddition, the paired molecules are bound together into a dimer through a ring made of four carbon atoms. Slight heating reverses this process.
“By using X-ray crystallographic analysis, we were able to determine the precise spatial arrangement (stereostructure) of a phylloxanthobilins and the hydrogen-bonded pair structure they adopt when crystallized,” reports Kräutler. “The fascinating chemistry of these substances also suggests that phyllobilins may have important, unknown physiological roles, possibly in the photoregulation of plants. Our new insights will help to elucidate this role.”
- Chlorophyll-Derived Yellow Phyllobilins of Higher Plants are Medium-Responsive, Chiral Photoswitches,
Chengjie Li, Klaus Wurst, Steffen Jockusch, Karl Gruber, Maren Podewitz, Klaus R. Liedl, Bernhard Kräutler,
Angew. Chem. Int. Ed. 2016.
DOI: 10.1002/anie.201609481