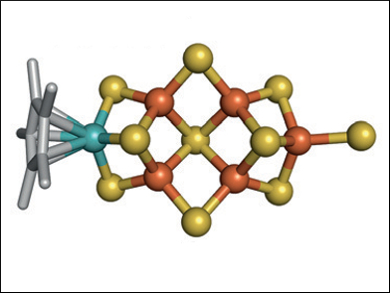
At the heart of the enzyme nitrogenase, there is a cofactor with a [MoFe7S9C] core that can perform challenging chemical conversions, such as N2 to NH3 and CO2 to CH4. Even when removed from the protein, the intact cofactor can catalyze reductions, which suggests that the structure of this cofactor is critical for the enzyme’s reactivity. However, the synthesis of clusters that mimic the asymmetric cofactor’s unique structure and function is difficult.
Yasuhiro Ohki, Kazuyuki Tatsumi, Nagoya University, Japan, Yilin Hu, Markus W. Ribbe, University of California, Irvine, USA, and colleagues have synthesized a synthetic cluster that has Mo and Fe atoms at the opposite ends in positions analogous to those of Mo and Fe in the cofactor. The team used [Cp*MoS3]− as a molybdenum source and combined it with a mixture of FeCl2 and [NEt4][SH] to prepare the cluster (pictured).
Both the [MoFe5S9]-core analogue and the cofactor can catalyze the reduction of C1 substrates (CN–, CO, and CO2) in the presence of protons and an electron source to rapidly generate short-chain hydrocarbons spanning from a length of C1 (CH4) to C5 (pentane). The reduction using the synthesized cluster is a method for converting greenhouse gases into renewable hydrocarbon fuels. The synthetic approach used to make the cluster could be used to prepare other heterometallic compounds.
- Structure and Reactivity of an Asymmetric Synthetic Mimic of Nitrogenase Cofactor,
Kazuki Tanifuji, Nathaniel Sickerman, Chi Chung Lee, Takayuki Nagasawa, Kosuke Miyazaki, Yasuhiro Ohki, Kazuyuki Tatsumi, Yilin Hu, Markus W. Ribbe,
Angew. Chem. Int. Ed. 2016.
DOI: 10.1002/anie.201608806