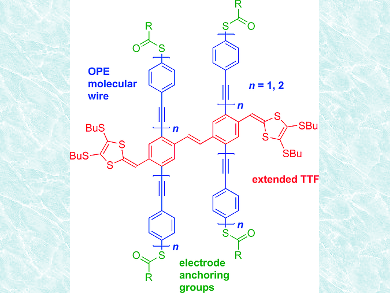
Oligo(phenyleneethynylene)s (OPEs) have been widely studied as molecular wires for electron transport in molecular electronics. A simple method to modify their properties is the introduction of electron-donating and/or -accepting units. In that context, the redox-active tetrathiafulvalene (TTF) is a promising electron donor.
Brøndsted Nielsen and colleagues, University of Copenhagen, Denmark, have prepared two “H-cruciforms” based on a central stilbene unit (pictured) by phosphite-mediated coupling reactions under microwave heating. Each of the stilbene rings is the central part of a OPE wire with three or five phenylene units (OPE3 or OPE5), which constitutes to the H-cruciform motif. Additionally, each stilbene ring is substituted with a dithiafulvalene unit, which can be viewed as an extended TTF motif orthogonal to the OPE wires.
Simplified, the H-cruciforms can be considered as three separate units, one OPV2-TTF [OPV = oligo(phenylenevinylene)] and two OPEs, which is indicated by characteristic absorptions for OPEs and extended TTFs in the UV/Vis spectra. Oxidation potentials of the compounds were obtained by cyclic voltammetry at high scan rates to suppress intermolecular reactions. Future investigations will focus on the extension of the orthogonal OPV unit and on single-molecule conductance studies.
- Synthesis of Covalently Linked Oligo(phenyleneethynylene) Wires Incorporating Dithiafulvene Units: Redox-Active “H-Cruciforms”,
Frederik Praestholm Jørgensen, Johannes F. Petersen, Cecilie Lindholm Andersen, Anders B. Skov, Martyn Jevric, Ole Hammerich, Mogens Brøndsted Nielsen,
Eur. J. Org.Chem. 2017.
DOI: 10.1002/ejoc.201601367