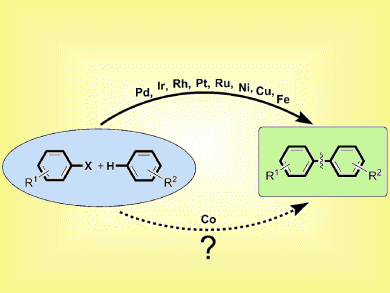
Palladium, nickel, and copper have received much attention as catalysts for cross-coupling reactions that form new C–C bonds. Cobalt, on the other hand, is relatively unexplored as a cross-coupling catalyst despite its lower toxicity and lower cost. Similarly, unactivated arenes, such as benzene, are not often used due to their low reactivity.
Aiwen Lei and co-workers, Wuhan University, China, have shown that the direct arylation of unactivated benzene can be performed with cobalt. Inexpensive and easy to handle [Co(acac)3] was used with lithium bis(trimethylsilyl)amide as a base. The reaction showed high efficiency, even with unactivated arenes and without extra ligands, and led to various biaryl products in excellent yields. In addition, an intramolecular direct arylation was also performed.
- Cobalt-Catalyzed Direct Arylation of Unactivated Arenes with Aryl Halides
W. Liu, H. Cao, J. Xin, L. Jin, A. Lei,
Chem. Eur. J. 2011.
DOI: 10.1002/chem.201002290