Oxygen-Sensing Materials
Monitoring the amount of oxygen in living tissues accurately is a valuable tool in biomedical science, because it enables the elucidation of the course of metabolic processes or the detection of diseases or anomalies. Metal complexes that absorb and emit light are useful as sensors, and metal complexes of porphyrins and their derivatives are especially good candidates for such applications, as the porphyrin macrocycle can easily be modified. Sergey M. Borisov and his co-workers at Graz University of Technology, Austria, developed strongly phosphorescent porphyrin complexes of iridium(III), which were applied as dyes in advanced optical oxygen-sensing materials.
Broader Absorption with Iridium
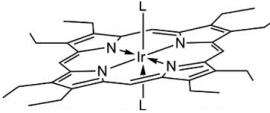
Photophysical properties of porphyrin complexes of metals such as palladium or platinum have been studied before; however, there are fewer studies on iridium complexes, which are more difficult to synthesize. The absorption bands of iridium complexes are broader and are shifted to lower wavelengths in comparison to those of their platinum analogues. This enables them to be excited by visible light. Furthermore, iridium(III) is hexacoordinate, which opens up the added possibility of introducing axial ligands directly on the metal instead of modifying the porphyrin macrocycle, in contrast to the square-planar platinum(II) and palladium(II) analogues. A π-extended iridium(III)–benzoporphyrin and four iridium(III)–octaethylporphyrin complexes with high room-temperature phosphorescence quantum yields of up to 30 % were synthesized. Axial ligands were used to change their solubility or to introduce binding groups. In this way, the complexes were rendered soluble in organic solvents, and they were incorporated into polystyrene or other polymers to yield oxygen sensors. In addition, other axial ligands, such as an imidazole ligand bearing a carboxyl group, were used to make the complexes soluble in polar solvents such as ethanol and even in aqueous buffer at physiological pH, which enabled coupling to biomolecules such as proteins, antibodies, or lipids, as demonstrated by coupling to bovine serum albumin.
Tunable Properties
The importance of these new compounds is their tunable photophysical properties and versatility, as demonstrated by their application as a water-soluble oxygen probe (by staining bovine serum albumin) and a trace oxygen sensor (by coupling to amino-modified silica gel). The obtained sensor is sensitive to small oxygen concentrations and features a highly linear calibration plot. The new dyes are particularly promising as indicators for oxygen sensors with tailor-made sensitivity.
Images: (c) Wiley-VCH
- Strongly Phosphorescent Iridium(III)–Porphyrins—New Oxygen Indicators with Tuneable Photophysical Properties and Functionalities
K. Koren, S. M. Borisov, R. Saf, I. Klimant,
Eur. J. Inorg. Chem. 2011.
DOI: 10.1002/ejic.201100089


