Sparklers are hand-held fireworks that burn slowly while emitting sparks.
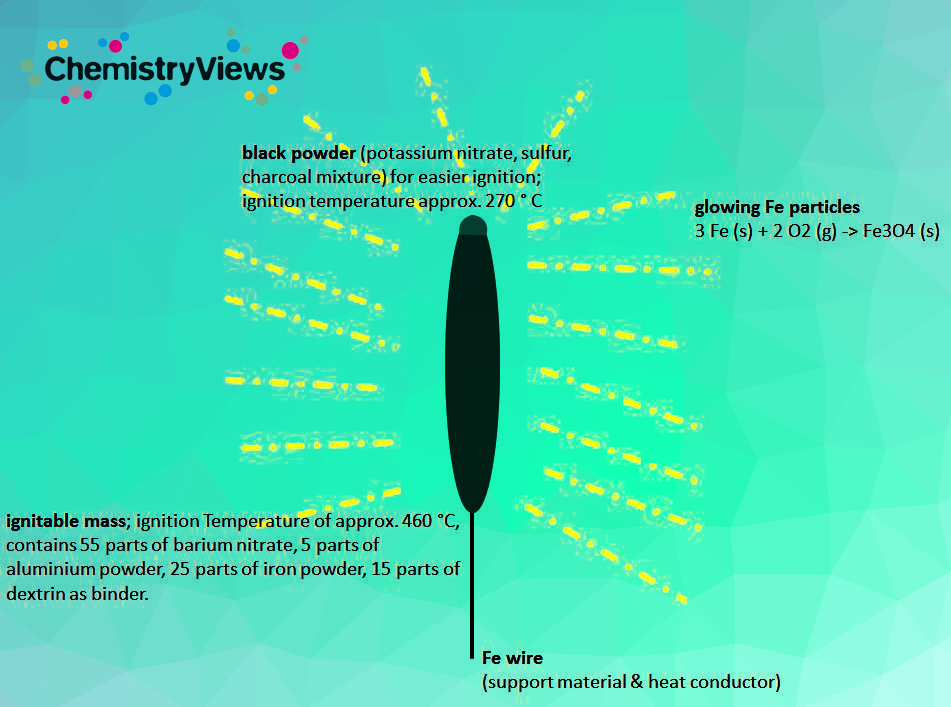
Typical redox-reactions taking place during ignation include:
10 Al + 3 Ba (NO3)2 → 3 BaO + 3 N2 + 5 Al2O3
15 Fe + 4 Ba (NO3)2 → 4 BaO + 4 N2 + 5 Fe3O4
3 Fe (s) + 2 O2 (g) → Fe3O4 (s)
You should not burn a sparkler indoors because part of the nitrate nitrogen is not completely reduced to the oxidation stage zero, but released as nitrogen dioxide:
2 Al + 3 Ba (NO3)2 → 3 BaO + 6 NO2 (g) + Al2O3
Reference
- Chemie der Wunderkerze – ein Thema nicht nur in der Weihnachtszeit,
Christina Martin, Tönjes de Vries,
Chemkon 2014, 11 (1), 13–20.
https:doi.org/10.1002/ckon.200410002