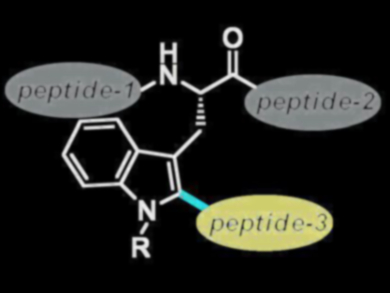
The chemoselective late-stage modification of peptides is used for the preparation of new bioactive compounds and for the study of protein structure and function. Palladium-catalyzed cross-coupling reactions can be used to modify peptides, but the process is often lengthy and can lead to byproducts.
Lutz Ackermann, University of Göttingen, Germany, and colleagues have developed a strategy for the chemoselective modification of peptides by ruthenium-catalyzed C‒H activation. The team used a ruthenium(II)biscarboxylate catalyst system consisting of [RuCl2(p-cymene)]2, K3PO4, and F5C6CO2H to modify peptides and amino acids by C−H arylation. The reaction proceeds under racemization-free conditions.
The ruthenium(II)biscarboxylate catalyst proved tolerant to water as well as to various amino acids. The method can be used to introduce fluorescence labels or to bind peptides together (pictured).
- Bioorthogonal Diversification of Peptides through Selective Ruthenium(II)-Catalyzed C–H Activation,
Alexandra Schischko, Hongjun Ren, Nikolaos Kaplaneris, Lutz Ackermann,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201609631