Metal-organic frameworks (MOFs) and porous organic frameworks (POFs) can be used for gas sorption (e.g., hydrogen, methane, or carbon dioxide), catalysis, and energy applications (Li-batteries, solar cells, fuel cells), amongst others. The majority of these known porous soft frameworks contain organic linker units.
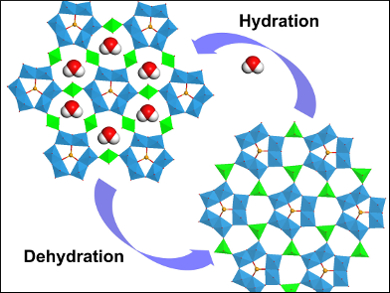
Zhenxin Zhang, Kanagawa University, Japan, and colleagues have created a much rarer, purely inorganic, soft framework material. The advantages of a framework without the organic linkers are a greater stability, together with potential redox properties, and a more complex chemical makeup. The material, denoted CoWSeO·H2O, is formed from tungsten selenide wires, which are linked through cobalt ions to form the three dimensional framework.
The material has a particular affinity for water, and for every mole of compound, can absorb eleven moles of water. This absorption is accompanied by a structural change, shifting the arrangement of the wires to each other, through an alteration in cobalt coordination. This change is fully reversible upon desorption of the water. The rarity of purely inorganic soft frameworks makes this an important result for further understanding the materials science of such compounds. The researchers envisage further applications as this class of materials is expanded.
- The Assembly of an All-Inorganic Porous Soft Framework from Metal Oxide Molecular Nanowires,
Zhenxin Zhang, Masahiro Sadakane, Shin-ichiro Noro, Norihito Hiyoshi, Akihiro Yoshida, Michikazu Hara, Wataru Ueda,
Chem. Eur. J. 2017.
DOI: 10.1002/chem.201605258