The surface-sensitive quantitative spectroscopic technique X-ray photoelectron spectroscopy (XPS) can be used to study what elements are present at a surface and in which relative quantities. Materials are irradiated with a beam of X-ray radiation and the kinetic energy and number of electrons that escape from the surface are analyzed. Metals, alloys, semiconductors, glasses, polymers, organics, ceramics, oils, etc. can be detected down to levels of parts per thousand. Hydrogen and helium cannot be detected.
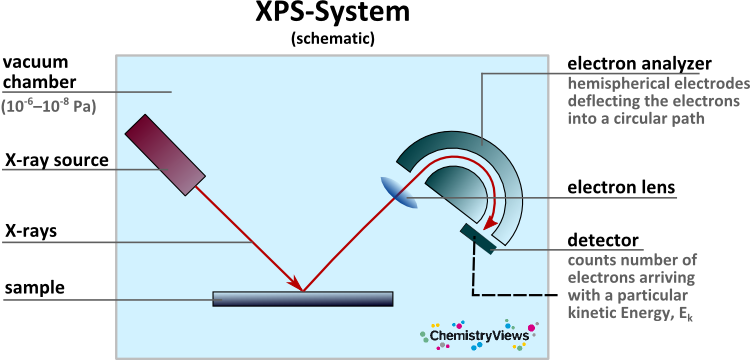
Electron’s Binding Energy, EB
EB = hν – Ek – W
hν = known energy of incident X-ray
W = spectrometer work function
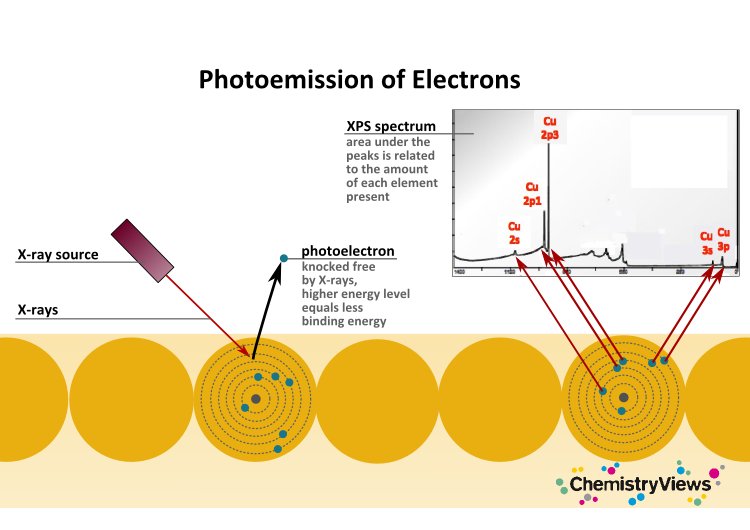
Electrons undergo more scattering if they originate from deeper in a sample. This limits how deep within a sample the photoelectrons can come from. This makes XPS such a surface-sensitive technique.
Advantages/Disadvantages
+ no other analytical technique provides same useful combination of surface sensitivity; quantifiable elemental and chemical information
– spatial resolution of typically 1 mm; can be improved to 10 µm or less in small area XPS and XPS imaging
- Thermo Scientific, X-ray Photoelectron Spectroscopy, Essential Knowledge Briefings, Microscopy and Analysis 2016. Link