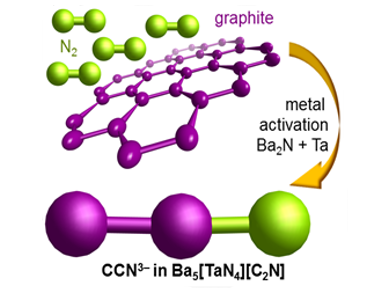
The acetonitriletriide anion CCN3– is the fully deprotonated form of acetonitrile and is isoelectronic to CO2. Michael Ruck and Eike Brunner, TU Dresden, Germany, and Peter Höhn, MPI for Chemical Physics of Solids, Germany, and their coworkers stabilized CCN3– in the bulk host framework of a Ba5[TaN4][C2N] nitridometalate via a one-pot synthesis from the elements under moderate conditions (650 °C). This route is unparalleled; typical synthetic routes to N-containing organic compounds or carbon nitrides use reactive precursors like ammonia for C–N bond-formation
The molecular structure was verified by various methods such as NMR and vibrational spectroscopy as well as quantum chemical calculations. In contrast to acetonitrile (H3C−C≡N), the electron pairs of CNN3– are shifted towards two double bonds, that is, [C=C=N]3−.
CCN3– is the most recent example of electron-rich anions being stabilized within a combined framework of a nitridometalate structure and alkaline-earth cations. Other examples include cyanamide anions CN22– as well as highly reduced cyano metalates such as [Co(CN)3]6–. According to the researchers, their work might pave the way to more examples of highly charged anions as well as to a general stabilization concept.
- The Triply Deprotonated Acetonitrile Anion CCN3− Stabilized in a Solid,
Franziska Jach, Stephan Ingmar Brückner, Alexander Ovchinnikov, Anna Isaeva, Matej Bobnar, Matthias Friedrich Groh, Eike Brunner, Peter Höhn, Michael Ruck,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201611177