The azide anion is a versatile and very useful reagent in chemical synthesis. Functioning as a nucleophile, it provides access to a variety of nitrogen functionalities after addition to an electrophilic center. In many cases, sodium azide is used due to its availability and relative safety. Only very few other main- or transition-metal azides have been reported, due in part to their instability and explosive nature.
Alexey Sukhorukov, N. D. Zelinsky Institute of Organic Chemistry, Moscow, Russia, and colleagues studied the selective reactions of in-situ generated metal azides on N,N-bis(oxy)enamines. By combining readily generated bis(oxy)enamine substrates with sodium azide and one of a variety of metal salts (including Mg, Cu, Zn, Co, and Ni), the researchers showed that the metal cation selectively alters the mechanism to favor the formation of specific organoazide products.
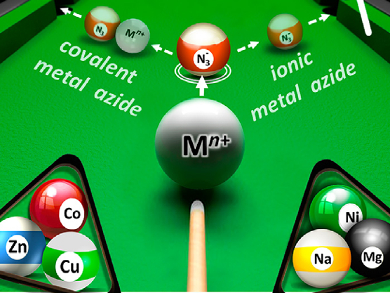
In all cases, cleavage of the N-silyl ethers in the starting material occurred, but via two different routes. Ionic metal azides attacked the silicon atom directly, producing the conjugated nitrosoalkenes, whereas covalently bound metal azides transferred N3 to a β-carbon through a Lewis acid-assisted substitution reaction. The results show that such in-situ generated metal azide species can be stable and useful reagents in the synthesis of azide products. The chemical properties can be readily tuned by the nature of the metal involved as well as by the N3/M ratio. The method could be used in synthetic routes for precursors of stereochemically complex products, such as unsymmetrically substituted diaminoalcohols, which are difficult to obtain.
- Divergent Reactivity of In Situ Generated Metal Azides: Reaction with N,N-Bis(oxy)enamines as a Case Study,
Petr A. Zhmurov, Yulia A. Khoroshutina, Roman A. Novikov, Ivan S. Golovanov, Alexey Yu. Sukhorukov, Sema L. Ioffe,
Chem. Eur. J. 2017.
DOI: 10.1002/chem.201605390