John Cowdery Kendrew was born in Oxford, UK, on March 24, 1917. He studied chemistry at Cambridge University and graduated in 1939. Before World War II, he had started a doctorate in physical chemistry. During the war, Kendrew worked on radar research and during his missions, he met the X-ray crystallography pioneer John Desmond Bernal and Linus Pauling, who had done research on protein structures. These connections changed the direction of Kendrew’s research: After the war, he switched fields and joined Max Perutz’s group at the Medical Research Council (MRC) Unit of Cavendish Laboratory, Cambridge University, to study protein structures using X-ray crystallography.
Perutz was trying to find the structure of the protein hemoglobin at the time. Hemoglobin is responsible for the transport of oxygen in blood. Kendrew was assigned to finding the structure of the much smaller myoglobin, which stores oxygen in muscles. His myoglobin samples first came from horse heart, but the protein gave only small crystals, which did not allow him to solve the structure. He then switched to whale meat as a source, which is rich in myoglobin since whales have to store large amounts of oxygen during diving. This myoglobin gave crystals of sufficient size.
To solve the phase problem of X-ray crystallography, researchers at the time often had to make educated guesses about the structure of a molecule. This was feasible for smaller compounds, but not for large proteins. Based on work by Perutz, Kendrew chemically modified myoglobin and added heavy metal atoms to different parts of the molecule. He then used the positions of those metal atoms as reference points to solve the phase problem and find the structure of the protein.
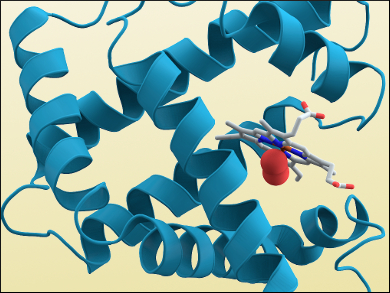
Another problem was the large amount of data generated by the experiments, which needed to be analyzed. Kendrew used one of the earliest computers at a British university to tackle this problem: the “electronic delay storage automatic calculator” (EDSAC I) designed by computer scientist Maurice Wilkes at the University of Cambridge’s Mathematical Laboratory. He arrived at a structure for myoglobin with a resolution of 6 Å in 1957. A more detailed structure which showed the protein’s alpha helices (pictured above) was obtained in 1960.
Max Perutz had completed his work on the structure of hemoglobin in 1959, and together, he and Kendrew received the Nobel Prize in Chemistry in 1962 “for their studies of the structures of globular proteins”. Kendrew was committed to promoting molecular biology and was founder and first Editor-in-Chief of the Journal of Molecular Biology, as well as co-founder and first General Director (1975–1982) of the European Molecular Biology Laboratory (EMBL) in Heidelberg, Germany. He died on August 23, 1997, in Cambridge.
John Kendrew is the answer to Guess the Chemist (63).
Sources
- Sir John Cowdery Kendrew. 24 March 1917 – 23 August 1997,
K.C. Holmes,
Biogr. Mems. Fell. R. Soc. 2001, 47, 311–332.
DOI: 10.1098/rsbm.2001.0018 - John C. Kendrew Dies at 80; Biochemist Won Nobel in ’62,
Wolfgang Saxon,
The New York Times, August 30, 1997. - John C. Kendrew – Biographical,
in Nobel Lectures, Chemistry 1942–1962,
Elsevier Publishing Company, Amsterdam, 1964.
Selected Publications by John Kendrew
- Comparison Between the Amino-Acid Sequences of Sperm Whale Myoglobin and of Human Hæmoglobin,
H. C. Watson, J. C. Kendrew,
Nature 1961, 190, 670–672.
DOI: 10.1038/190670a0 - A Partial Determination by X-ray Methods, and its Correlation with Chemical Data,
J. C. Kendrew, H. C. Watson, B. E. Strandberg, R. E. Dickerson, D. C. Phillips, V. C. Shore,
Nature 1961, 190, 666–670.
DOI: 10.1038/190666a0 - Structure of Myoglobin: A Three-Dimensional Fourier Synthesis at 2 Å. Resolution,
J. C. Kendrew, R. E. Dickerson, B. E. Strandberg, R. G. Hart, D. R. Davies, D. C. Phillips, V. C. Shore,
Nature 1960, 185, 422–427.
DOI: 10.1038/185422a0 - The Crystal Structure of Myoglobin. V. A Low-Resolution Three-Dimensional Fourier Synthesis of Sperm-Whale Myoglobin Crystals,
G. Bodo, H. M. Dintzis, J. C. Kendrew, H. W. Wyckoff,
Proc. R. Soc. A 1959, 253, 70–102.
DOI: 10.1098/rspa.1959.0179 - A Three-Dimensional Model of the Myoglobin Molecule Obtained by X-Ray Analysis,
J. C. Kendrew, G. Bodo, H. M. Dintzis, R. G. Parrish, H. Wyckoff, D. C. Phillips,
Nature 1958, 181, 662–666.
DOI: 10.1038/181662a0 - Electron Spin Resonance in Myoglobin and Hæmoglobin: Orientation of the Hæm Group in Myoglobin and its Relation to the Polypeptide Chain Direction,
D. J. E. Ingram, J. C. Kendrew,
Nature 1956, 178, 905–906.
DOI: 10.1038/178905a0 - Imidazole Complexes of Myoglobin and the Position of the Hæm Group,
J. C. Kendrew, R. G. Parrish,
Nature 1955, 175, 206–207.
DOI: 10.1038/175206b0 - The Species Specificity of Myoglobin,
J. C. Kendrew, R. G. Parrish, J. R. Marrack, E. S. Orlans,
Nature 1954, 174, 946–949.
DOI: 10.1038/174946a0 - The computation of Fourier synthesis with a digital electronic calculating machine,
J. M. Bennett, J. C. Kendrew,
Acta Crystallogr. 1952, 5, 109–116.
DOI: 10.1107/S0365110X52000228


I am writing this note, as a mark of respect to my Guru Sir John Kendrew. Actually, I wanted to work in his group, after my M.Sc. in Biochemistry 1970. But he declined because I did not have sufficient knowledge of Physics and Maths. However, he did encourage me – between 1970 and 74 – and that is why I could find my field, physics education, in which I am working alone for the last 40 years. I wrote an essay in his memory, title: From Kendrew to Newton: a journey to the centre of physics.