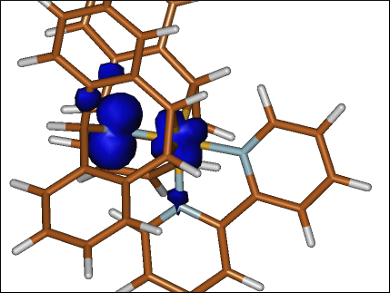
Metalloradicals have spin densities that are mostly confined to the metal center, but in some cases, the unpaired electron can be delocalized onto the ligand. These compounds show high reactivity and selectivity in various catalytic processes. For metal aminyl radical compounds, two resonance structures (metal amide and aminyl radical complex) are possible, which makes their classification challenging.
Bas de Bruin, University of Amsterdam, The Netherlands, Monica Trincado and Hansjörg Grützmacher, Swiss Federal Institute of Technology (ETH) Zurich, Switzerland, and colleagues have used a combination of structural analysis, electron paramagnetic resonance (EPR) spectroscopy, and density functional (DFT) calculations to elucidate the structure of the complex [Co(trop2N)(bpy)]PF6 (complex pictured, trop = 5H-dibenzo[a,d]cyclohepten-5-yl, bpy = 2,2′-bipyridine).
The team determined that upon formation of the radical through oxidation, the electron is removed from a Co–N π-antibonding orbital. The oxidation mainly occurs at the N atom, with some delocalization of the positive charge on the Co center. Based on DFT calculations, the spin density of the radical complex is strongly delocalized. A mixture between the CoII amido form and the CoI-aminyl radical forms as limiting resonance structures. However, the N atom has a slightly higher spin population than the Co center, indicating that the CoI-aminyl radical slightly prevails over the CoII amido form.
- A Stable Aminyl Radical Coordinated to Cobalt,
Rafael E. Rodríguez-Lugo, Bas de Bruin, Monica Trincado, Hansjörg Grützmacher,
Chem. Eur. J. 2017.
DOI: 10.1002/chem.201605624