The removal of pollutants by means of photocatalysis under solar light irradiation has become a promising technology. Considering some drawbacks of the commercially used TiO2 catalyst, e.g., a high recombination rate of charge carriers, developing new photocatalysts to meet environmental requirements is a subject of ongoing research.
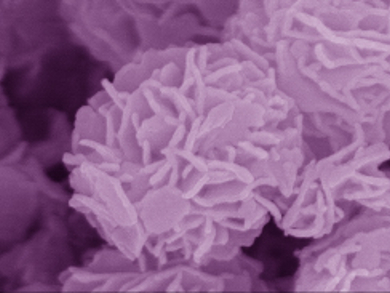
Qiaofeng Han, Junwu Zhu, and co-workers from Nanjing University of Science and Technology, China, have developed a catalyst that is made of Bi2O2(OH)(NO3) (BION) and forms a microstructure that resembles a flower (pictured). The synthesis involves the hydrothermal treatment of bismuth nitrate, which is pretreated with acetic acid.
The BION flowers were tested for their ability to photodegrade the rhodamine B dye. The reaction is very quick and reaches 100 % conversion within 10 minutes – a rate about seven times faster than achieved with the commercial TiO2 catalyst P25. Further investigation revealed that photo-induced holes were the main reactive species for the catalytic reaction. The result provides new insights into the design of nontoxic bismuth-based photocatalysts with good performance.
- Synthesis of Unique Flowerlike Bi2O2(OH)(NO3) Hierarchical Microstructures with High Surface Area and Superior Photocatalytic Performance,
Qiaofeng Han, Jiawei Pang, Xin Wang, Xiaodong Wu, Junwu Zhu,
Chem. Eur. J. 2017.
DOI: 10.1002/chem.201604085