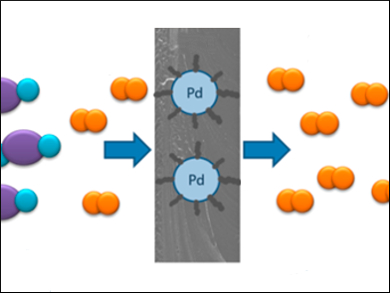
Purified hydrogen is required in many thermochemical, electrolytic, and biological processes. The most common purification method is membrane separation. This technique offers low energy consumption, a small footprint, and easy operation. However, the separation of mixtures of H2 and carbon dioxide can be particularly challenging. Palladium membranes are the most efficient in this regard, but they are costly. A possible alternative is the use of mixed-matrix membranes (MMMs) based on a polymeric membrane with incorporated Pd nanoparticles.
Cho Peng Leo, Universiti Sains Malaysia, Gelugor, and colleagues have prepared polybenzimidazole (PBI) membranes with different loadings of polyethylene glycol (PEG)-stabilized Pd nanoparticles. The team synthesized the palladium nanoparticles using PdCl2 as the Pd precursor and PEG as both reducing agent and stabilizer. The nanoparticles were then added to the polymer solution polymer, which was cast onto glass plates and solidified by evaporation of the solvent. The MMMs were characterized by, e.g., scanning electron microscopy and energy dispersive X-ray analysis.
The gas selectivity of the MMMs for H2 over CO2 was measured at 150 °C and 200 °C under a feed pressure of 5 bar. The material’s H2/CO2 selectivity at 200 °C is 18.6. Temperature-programmed reduction analysis showed that, unlike pure Pd membranes, the MMMs already start interacting with H2 at lower temperatures. Only a small amount of Pd nanoparticles is needed to significantly increase the H2/CO2 selectivity at elevated operating temperature compared to pure PBI membranes.
- Hydrogen Purification Using Polybenzimidazole Mixed-Matrix Membranes with Stabilized Palladium Nanoparticles,
Hani Shazwani Mohd Suhaimi, Choe Peng Leo, Abdul Latif Ahmad,
Chem. Eng. Technol. 2017.
DOI: 10.1002/ceat.201600457