Polycyclic ring structures can be formed through cascade reactions. This typically involves the isomerization of alkyne species by π-electron conjugation towards a more electrophilic species. Gold metal sources are often used for such reactions, which have often been shown to form carbenic intermediates. These gold vinylidene species are short-lived and very difficult to characterize.
Wouter Debrouwer and Alois Fürstner, Max Planck Institute (MPI) for Coal Research, Mülheim, Germany, have discovered an unprecedented gold-catalyzed cyclization through an aldehyde group on a biaryl moiety. Although the aldehyde is not inherently nucleophilic enough to be attacked by the gold acetylide, the addition of a Lewis acid activated the aldehyde sufficiently to drive the process at low temperature.
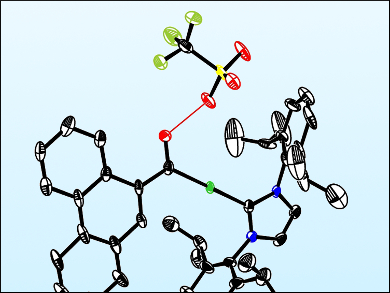
The resulting complex was characterized using NMR spectroscopy and X-ray crystallography. It is a previously unreported Fischer-type gold carbene species with anthracene and hydroxyl groups. This species is proposed to form when the alkynylgold group attacks the activated formyl substituent, which produces a transient gold vinylidene species. This discovery could pave the way towards a full characterization of reaction intermediates of this type, and thereby increase the understanding of the mechanism of metal acetylide cyclizations.
- Rearrangement of a Transient Gold Vinylidene into Gold Carbenes,
Wouter Debrouwer, Alois Fürstner,
Chem. Eur. J. 2017.
DOI: 10.1002/chem.201700326