The photophysical properties and molecular structures of triangulenes can be modified by substituting carbon atoms in the polybenzoid framework with alternate atoms. However, only a limited set of central and bridging heteroatoms is accommodated by conventional linear approaches to triangulene skeleton construction such as Friedel–Crafts or SNAr nucleophilic aromatic substitution reactions.
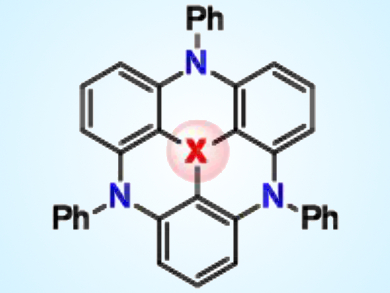
To address this issue, Takuji Hatakeyama, Kwansei Gakuin University, Sanda, Japan, and colleagues have developed a divergent synthesis approach. The team incorporated a boron, phosphorus, or silicon heteroatom into a nitrogen-containing macrocyclic precursor by electrophilic carbon–lithium and carbon–hydrogen bond substitutions. Boron-, phosphorus-, and silicon-centered 4,8,12-triazatriangulenes (pictured) with unusual properties were produced using this method.
The boron adduct displayed ambipolar charge-carrier transport properties suited to organic light-emitting diodes, whereas the phosphorus and silicon adducts assumed bowl-shaped structures with different depths. Substituted triangulenes could meet the demands of functional materials requiring versatile photophysical and supramolecular properties.
- Divergent Synthesis of Heteroatom-Centered 4,8,12-Triazatriangulenes,
Soichiro Nakatsuka, Hajime Gotoh, Keisuke Kinoshita, Nobuhiro Yasuda, Takuji Hatakeyama,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201701246



![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)