Oxygen atom transfer (OAT) is common in many biological transformations and synthetic organic methods, where high-valent metal-oxo species are key intermediates. The oxygen-evolving complex of photosystem II, a multinuclear enzyme active site responsible for biological water oxidation, is also proposed to contain reactive metal-oxo moieties. Investigating redox changes in synthetic clusters and their influence on OAT at remote metal centers can provide valuable information regarding structure-function relationships relevant to biological systems.
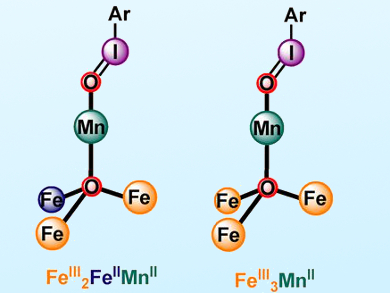
Theodor Agapie, California Institute of Technology (Caltech), Pasadena, CA, USA, and colleagues have prepared a series of metal clusters with oxidation states that differ by a single electron (FeIII2FeIIMnII vs. FeIII3MnII). Remarkable differences in the rate of intramolecular C−H bond oxygenation of 2-(tert-butylsulfonyl)-iodosobenzene (sPhIO) were observed for these clusters.
These differences were attributed to the reactivity of iodosobenzene-metal adducts. FeIII3MnII-OIsPh (pictured right) is one of the very few examples of such species characterized by single crystal X-ray diffraction. These results provide new insights regarding the effect of redox state and electron distribution within biomimetic transition-metal clusters on their reactivity.
- Accelerated Oxygen Atom Transfer and C−H Bond Oxygenation by Remote Redox Changes in Fe3Mn-Iodosobenzene Adducts,
Graham de Ruiter, Kurtis M. Carsch, Sheraz Gul, Ruchira Chatterjee, Niklas B. Thompson, Michael K. Takase, Junko Yano, Theodor Agapie,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201701319