Mechanically interlocked molecules (MIMs) such as rotaxanes and catenanes have progressed from compounds of aesthetic interest and curiosity to an active area of contemporary chemical research, with applications in nanotechnology, drug delivery, catalysis, materials science, and molecular recognition.
Paul Beer, University of Oxford, UK, and colleagues synthesized a family of neutral [2]rotaxanes containing the halogen-binding (XB) donor group iodotriazole, which was integrated into the macrocycle and axle components using an active-metal template synthetic strategy. The team attached 2 XB donor groups either meta– or ortho-positioned to each other on a phenyl ring spacer in the macrocycle, but none to the axle. Using NMR spectroscopy, they showed that the meta-arranged molecules can bind anions (Cl–, Br–, I–, and SO42–) more strongly than the ortho isomer. Donor group flexibility and steric hindrance may play a role in the anion binding affinity of these MIMs.
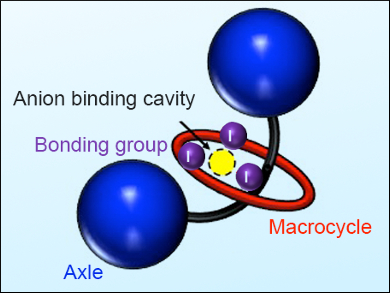
Using the same macrocycle, the researchers then added a third and fourth XB donor groups in the axle (example pictured) and showed increased halogen binding for both MIMs. However, non-spherical anions such as sulfates, azides, acetates, or nitrates did not bind to the molecule with four XB groups, which are thus selective towards halogens. This study opens up new possibilities for the design and preparation of elaborate XB-interlocked receptors capable of strong anion binding for various analytical and nanotechnological applications.
- Strong and Selective Halide Anion Binding by Neutral Halogen-Bonding [2]Rotaxanes in Wet Organic Solvents,
Jason Y. C. Lim, Thanthapatra Bunchuay, Paul D. Beer,
Chem. Eur. J. 2017, 23, 4700–4707.
DOI: 10.1002/chem.201700030