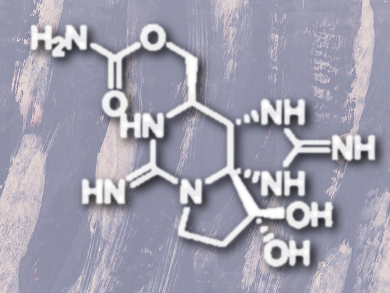
Shellfish Toxins
The chemical space of natural compounds is vast, but it is always interesting when a new skeleton emerges from an unusual source, especially if the natural product in question is toxic. One such compound, saxitoxin (STX, pictured) was first isolated from toxic Alaskan butter clams (Saxidomus giganteus) and both itself and its analogs are well-known paralytic shellfish toxins. They are biosynthesized by several species of prokaryotic freshwater blue-green algae (formerly known as cyanobacteria) and eukaryotic marine dinoflagellates, which are ingested by the clams and other shellfish. The compounds’ toxicity is based on their ability to potently and selectively inhibiting voltage-gated sodium channels in the body’s cells.
Unusually for inhibitors of such complex proteins, these compounds are not peptides nor protein fragments, but small molecules – albeit with complicated structures. Indeed, STX and its chemical cousins, of which there are more than fifty, has a unique tricyclic structure, two guanidinium moieties, multiple stereogenic carbon centers, and several heteroatoms. Researchers have attempted to unravel the biosynthetic schemes used by cyanobacteria and dinoflagellates to produce these compounds.
Key Biosynthetic Intermediates
Mari Yotsu-Yamashita, Tohoku University, Sendai, Japan, and colleagues have identified biosynthetic intermediates at the early stages of saxitoxin synthesis in microorganisms, using synthesis and liquid chromatography coupled mass spectrometry (LC/MS) techniques.
The presence of some of these intermediates had been proposed on the basis of genetic techniques [1]. However, getting to the heart of the tricyclic skeleton and its formation had not been feasible with these methods. Another suggested biosynthetic pathway begins with a Claisen condensation of arginine and acetyl-CoA, followed by decarboxylation, cyclization, and amidinylation to form first a bicyclic structure and then the tricyclic skeleton [2]. Other likely routes have been proposed since, and a set of 14 genes at the core of the biosynthesis have been picked out from cyanobacteria that produce these paralytic shellfish toxins.
The researchers have screened for unidentified intermediates by analyzing the results from previous incorporation experiments with nitrogen-15 labeled compounds. They then critically analyzed cell extracts to identify any putative intermediates using ultraperformance liquid chromatography coupled with mass spectrometry heat maps to home in on the compounds in a cultured sample and a control sample. The next step will be to synthesize an elusive pair of one bicyclic and one tricyclic compound the team is yet to make. “We plan to synthesize and identify them in these microorganisms to directly prove the proposed pathway,” Yotsu-Yamashita told ChemViews Magazine.
Possible Antidotes and New Structures
One has to imagine that the eventual unraveling of the complete biosynthetic scheme is only a matter of time. Its solution might then facilitate the synthesis of these and related compounds in the laboratory, which might find themselves at the heart of research into an antidote for paralytic shellfish poisoning. After all, saxitoxins can have an enormous impact on the seafood industry as mussels, clams, oysters, and scallops are all frequently contaminated with toxic algae leading to temporary bans on the commercial and recreational harvesting of these creatures in coastal waters around the world from the northeastern and western USA and western Europe, to east Asia, Australia, New Zealand, and South Africa.
There is also the possibility of the research pointing to novel molecular structures and derivatives that would not be toxic but might have pharmaceutical activity in modulating the properties and behavior of cellular sodium channels or other proteins that go awry in disease.
- Synthesis and Identification of Key Biosynthetic Intermediates for the Formation of the Tricyclic Skeleton of Saxitoxin,
Shigeki Tsuchiya, Yuko Cho, Renpei Yoshioka, Keiichi Konoki, Kazuo Nagasawa, Yasukatsu Oshima, Mari Yotsu-Yamashita,
Angew. Chem. Int. Ed. 2017, 56, 5327–5331.
DOI: 10.1002/anie.201612461
References
- [1] Biosynthetic Intermediate Analysis and Functional Homology Reveal a Saxitoxin Gene Cluster in Cyanobacteria,
R. Kellmann, T. K. Mihali, Y. J. Jeon, R. Pickford, F. Pomati, B. A. Neilan,
Appl. Environ. Microbiol. 2008, 74, 4044–4053.
DOI: 10.1128/AEM.00353-08 - [2] Biosynthesis of saxitoxin analogs: the unexpected pathway,
Yuzuru Shimizu, Manuel Norte, Akira Hori, Almoursi Genenah, Masaru Kobayashi,
J. Am. Chem. Soc. 1984, 106, 6433–6434.
DOI: 10.1021/ja00333a062