Amides are important functional groups in organic chemistry and medicinal chemistry. Amide bonds are present in biomolecules such as proteins, natural products, pharmaceutically active compounds, agrochemicals, materials, etc. Typically, amides are prepared from carboxylic acids and amines using amide coupling reagents such as N,N-dicyclohexylcarbodiimide (DCC) or N,N-diisopropylcarbodiimide (DIC).
Transamidation reactions can be an alternative to provide quick access to different amides in the absence of amide coupling reagents. However, the direct transamidation of amides usually requires harsh reaction conditions due to the high stability of the C(=O)−N bond.
Jeyakumar Kandasamy, Indian Institute of Technology (BHU), Varanasi, India, and colleagues have developed a general procedure for the preparation of amides via transamidation reactions in the absence of catalysts and coupling reagents. The team first converted a range of secondary amides into the corresponding activated N-Cbz amides via a reaction with benzyl chloroformate (Cbz-Cl, pictured below on the left). Then, they used the resulting N-Cbz amides in transamidations with various primary and secondary amines in the presence of 1,8-diazabicyclo(5.4.0)undec-7-ene (DBU) at room temperature (pictured below on the right).
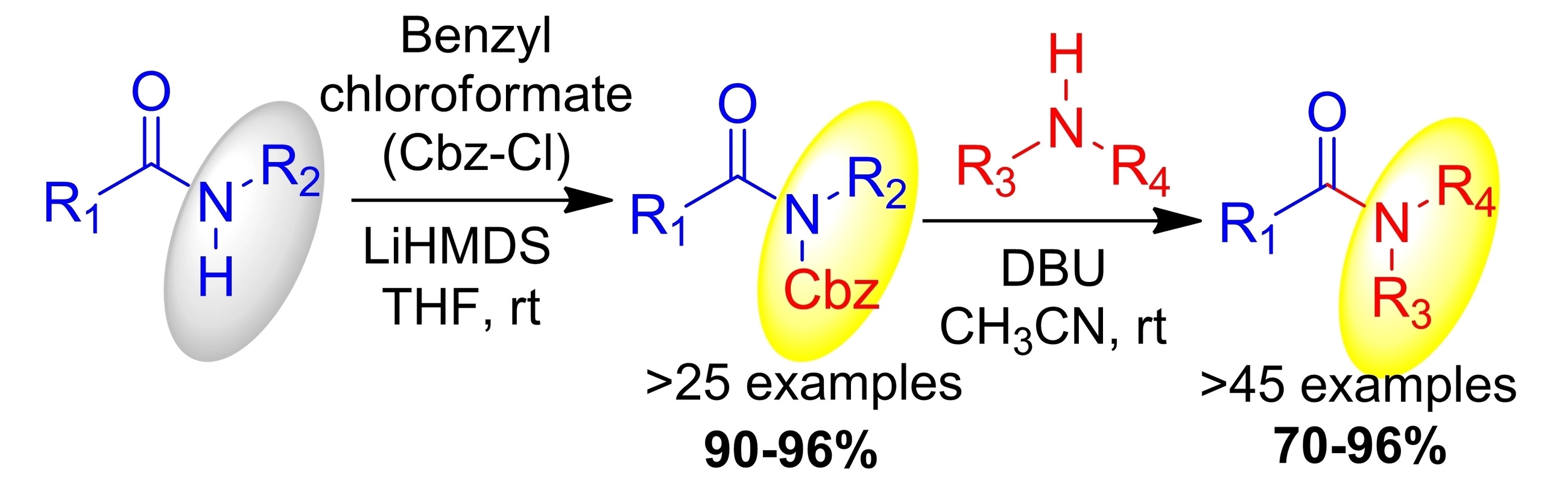
The desired amide products were obtained in good to excellent yields. The reaction has a broad substrate scope.
- Synthesis of N‐Cbz Amides and Their Applications in the Transamidation Reactions at Room Temperature ,
Popuri Sureshbabu, Sadaf Azeez, Koyal Pattanaik, Shahulhameed Sabiah, Jeyakumar Kandasamy,
Asian J. Org. Chem. 2022.
https://doi.org/10.1002/ajoc.202200076