The oxidation of alcohols is an important reaction in organic chemistry. Primary alcohols can be oxidized to form aldehydes and carboxylic acids; secondary alcohols can be oxidized to give ketones. Tertiary alcohols, in contrast, cannot be oxidized without breaking the molecule’s C–C bonds.
Oxidation Reactions
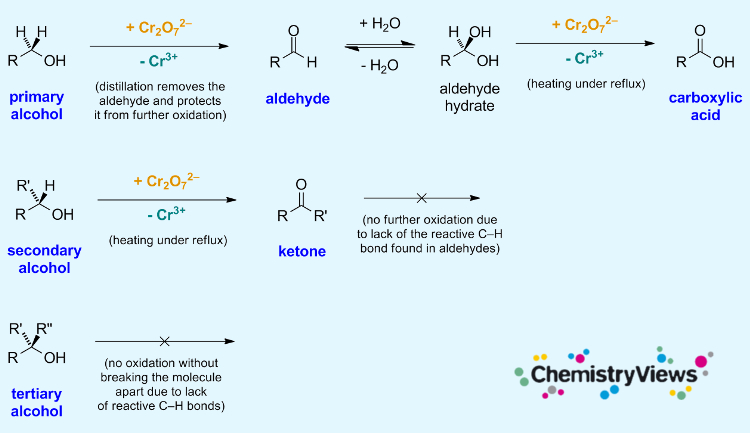
During the oxidation, the orange dichromate ion is reduced to the green Cr3+ ion. This can be used to detect alcohols.
Reactive C–H Bonds
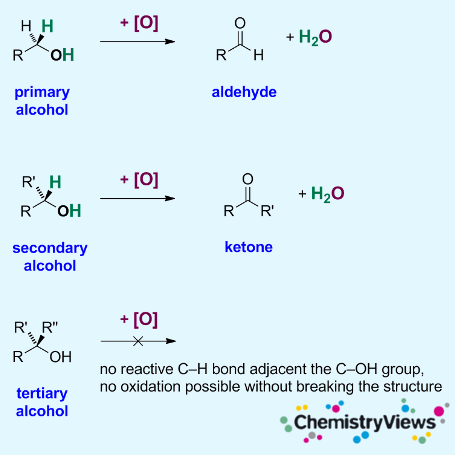
([O] = Oxidizer)
Reagents for Alcohol Oxidation
For the oxidation of primary alcohols to aldehydes
- Cr2O72– (dichromate)
- CrO3/pyridine (Collins reagent)
- Pyridinium chlorochromate (PCC)
- Pyridinium dichromate (PDC, Cornforth reagent)
- Dess–Martin periodinane
- Dimethylsulfoxide (DMSO)/oxalyl chloride (Swern oxidation)
- etc.
For the oxidation of secondary alcohols to ketones
- Cr2O72– (dichromate)
- CrO3/pyridine (Collins reagent)
- Pyridinium chlorochromate (PCC)
- Pyridinium dichromate (PDC, Cornforth reagent)
- Dess–Martin periodinane
- Dimethylsulfoxide (DMSO)/oxalyl chloride (Swern oxidation)
- CrO3/H2SO4/acetone (Jones oxidation)
- Aluminium isopropoxide/acetone (Oppenauer oxidation)
- etc.
For the direct oxidation of primary alcohols to carboxylic acids
- KMnO4 (potassium permanganate)
- RuO4 (ruthenium tetroxide)
- CrO3/H2SO4/acetone (Jones oxidation)
- etc.
Sources
- March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure,
Michael B. Smith,
Wiley 2013.
ISBN: 978-0-470-46259-1 - Lehrbuch der Organischen Chemie (in German),
Hans Beyer, Wolfgang Walter, Wittko Francke,
Hirzel 2004.
ISBN: 978-3-777-61221-8




Thank you to give me information.
Its appreciated as it is helpful to students and teachers as well.