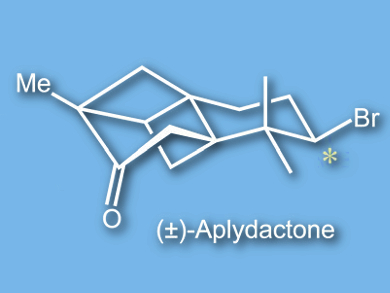
Aplydactone (pictured) is an unusual brominated sesquiterpene isolated from the sea hare Aplysia dactylomela. Its tetracyclic ring system contains a highly strained [2]-ladderane (two fused four-membered rings). To date, two total syntheses and a formal synthesis have been reported by the groups of Trauner, Burns, and Snyder, respectively [1–3].
Yandong Zhang, Xiamen University, China, and colleagues have developed a conceptually different approach to this marine natural product by relying on a novel C–H functionalization step. The key transformations include: 1) a [2+2] photocycloaddition followed by a Wolff ring contraction to establish the 6-4-4 tricycle, 2) an unusual transannular C–H insertion to set up the bridged cyclohexanone ring and a quaternary stereocenter between two quaternary carbon atoms, and 3) a hydrogen-atom-transfer hydrogenation to furnish the brominated stereocenter.
A finely tuned conformation of the α-diazoketone precursor is key for the C–H insertion. This strategy enabled a protecting-group-free synthesis of aplydactone in 12 steps from a simple enone compound. An efficient synthesis of this complex molecule may allow further studies of its biomedical properties.
- Total Synthesis of Aplydactone via a Conformationally Controlled C–H Functionalization,
Chenguang Liu, Renzhi Chen, Yang Shen, Zhanhao Liang, Yuhui Hua, Yandong Zhang,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201703803
References
- [1] A Synthesis of (±)-Aplydactone,
Robin Meier, Dirk Trauner,
Angew. Chem. Int. Ed. 2016, 55, 11251–11255.
DOI: 10.1002/anie.201604102 - [2] A Unified Approach for the Enantioselective Synthesis of the Brominated Chamigrene Sesquiterpenes,
Alexander J. Burckle, Vasil H. Vasilev, Noah Z. Burns,
Angew. Chem. Int. Ed. 2016, 55, 11476–11479.
DOI: 10.1002/anie.201605722 - [3] Strategies for the Total Synthesis of Diverse Bromo-Chamigrenes,
Minxing Shen, Manuel Kretschmer, Zachary G. Brill, Scott A. Snyder,
Org. Lett. 2016, 18, 5018–5021.
DOI: 10.1021/acs.orglett.6b02478