The Suzuki-Miyaura cross-coupling reaction is an essential procedure for the synthesis of many fine chemical and pharmaceutical products with biaryl moieties. Although this reaction has been well-developed, a number of substrates remain difficult to activate. Among these are heterocycles and heavily fluorinated aryl boronates, which typically require high temperatures and expensive additives to achieve decent yields.
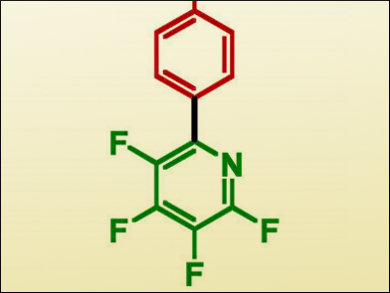
Thomas Braun, Humboldt-Universität zu Berlin, Germany, and colleagues, have achieved the coupling of a tetrafluorinated pyridine pinacolatoboranate with a number of protected bromophenylalanine derivatives (example product pictured). The complex [Pd(PiPr3)2] was reacted with the bromoarene substrate to give the oxidative-addition Pd(II) intermediate, which was isolated and characterized using X-ray crystallographic analysis. This proved to be successful as a catalyst, producing very good yields with a 10 mol % catalyst loading.
Low-temperature 31P{1H} NMR studies showed that the transmetallation step occurs through a rare palladium-fluorido intermediate after addition of NMe4F or CsF salts. Using this evidence, the reaction mechanism could be elucidated. This revealed that two catalytic cycles are possible, both of which occur through the palladium-fluorido intermediate, whereas transmetallation through a bromide intermediate is much less likely. This discovery reveals a number of key intermediates for this type of reaction, which could be essential for the design of new catalysts.
- Suzuki-Miyaura Cross-Coupling Reactions of Highly Fluorinated Arylboronic Esters: Catalytic Studies and Stoichiometric Model Reactions on the Transmetallation Step,
Johannes Kohlmann, Thomas Braun, Reik Laubenstein, Roy Herrmann,
Chem. Eur. J. 2017.
DOI: 10.1002/chem.201700549