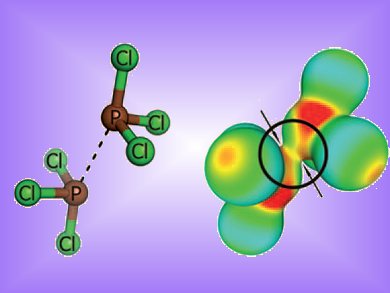
Self-assembly of molecules to form nanoparticles, monolayers, thin films and supramolecular structures, is often guided by hydrogen bonds or metal-ligand interactions. Chalcogen–chalcogen interactions or halogen bonds have also captured interest as connectors due to their unexpected strength. Eva Hey-Hawkins, Barbara Kirchner and co-workers, Universität Leipzig, Germany, now report that phosphorus dimers exhibit similar non-bonding interactions.
They investigated several examples of phosphorus-(III) compounds. In all cases the P•••P distance of the optimized structures was lower than the sum of the van der Waals radii of phosphorus (380 pm). The dissociation energy was up to 27.8 kJmol–1. This is comparable to a moderately strong hydrogen bond and could see them used as a molecular linker in self-assembling systems in the same way H-bonds are used.
- Pnicogen Bonds: A New Molecular Linker?
S. Zahn, R. Frank, E. Hey-Hawkins, B. Kirchner,
Chem. Eur. J. 2011.
DOI: 10.1002/chem.201002146