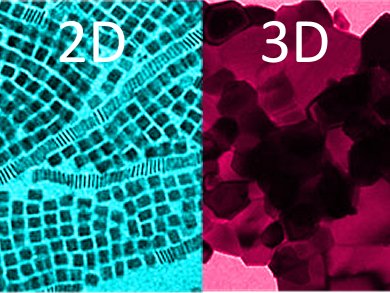
Shape Matters
Three dimensions are not necessarily better than two. Not where ceria is concerned, in any case. Ceria is an important catalyst. Because of its outstanding ability to store oxygen and release it, ceria is primarily used in oxidation reactions. Christopher B. Murray and a team at the University of Pennsylvania, USA, have now developed a simple synthetic technique to produce ceria in the form of nanoplates. As the researchers report in the journal Angewandte Chemie, these have proven to be better at storing oxygen than conventional three-dimensional nanoparticles.
In automotive catalytic converters, ceria helps to level out hydrocarbon spikes. It can also be used in the removal of soot from diesel exhaust and organic compounds from wastewater, for example. In fuel cells, ceria is used as a solid electrolyte. Cerium, a rare-earth metal, can easily switch between two different oxidation states (+IV and +III), so it undergoes a smooth transition between CeO2 and materials with a lower oxygen content. This makes ceria an ideal material for oxygen storage.
Controlling Morphology
Ceria can be produced as a nanomaterial in various different forms. Almost all of the previously described forms were three-dimensional. Murray’s team has now developed a handy method for the synthesis of two-dimensional nanoplates. Their synthetic technique is based on the thermal decomposition of cerium acetate at 320 to 330 °C. Critical to their success is the presence of a mineralization agent, which speeds up the crystallization process and controls the morphology. Depending on the reaction conditions, the researchers obtained either roughly square plates with a thickness of 2 nm and edges about 12 nm in length, or elongated plates with dimensions of about 14 x 152 nm.
To test the oxygen storage capacities of the various forms of ceria, the researchers established a very simple thermogravimetric test: They alternately exposed the samples to oxygen and hydrogen and recorded the change in mass due to oxygen absorption/emission. The nanoplates proved to be superior to the conventional particulate systems and displayed an oxygen capacity three to four times as high as that of conventional three-dimensional nanoparticles. The plates do have a higher surface-to-volume ratio than the three-dimensional particles but the uptake of oxygen in the body of the nanoplates is required to explain this magnitude of enhancement. Furthermore, not all surfaces of a ceria crystal are equally good for the absorption and emission of oxygen. It turns out that the platelet surfaces were of the right type.
- Synthesis and Oxygen Storage Capacity of 2-D Ceria Nanocrystals
D. Wang, Y. Kang, V. Doan-Nguyen, J. Chen, R. Küngas, N. L. Wieder, K. Bakhmutsky, R. J. Gorte, C. B. Murray,
Angew. Chem. Int. Ed. 2011, 50(19), 4378–4381.
DOI: 10.1002/anie.201101043