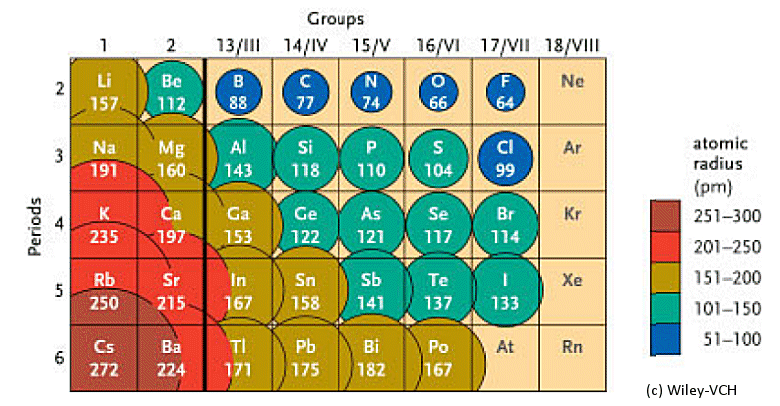
The atomic radii (averages) of the main group elements are shown in pm (10–15 m). The radii decrease from left to right across a period and increase down a group.
Taken from the book:
 World of the Elements, Elements of the World
World of the Elements, Elements of the World
Hans-Jürgen Quadbeck-Seeger
Wiley-VCH Verlag GmbH & Co, KGaA, Weinheim, 2007
Print ISBN: 9783527320653, Online ISBN: 9783527611577
DOI: 10.1002/9783527611577