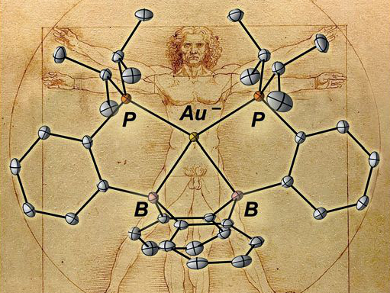
The transition metals are typically regarded as cation-forming elements. However, gold is a notable exception since it is stable as an isolated anion (Au– or auride). In addition to its unusual charge, the auride anion possesses 12 valence electrons, with fully filled 5d and 6s subshells. This unique electronic structure is made possible by the relativistic stabilization of the 6s orbital around the heavy gold nucleus. Although auride salts are known, the coordination chemistry of the auride ion in solution is basically unexplored.
W. Hill Harman, University of California, Riverside, USA, and colleagues have developed a molecular framework capable of stabilizing an auride equivalent. They achieved this by incorporating strong acceptor interactions into a ligand based on diboraanthracene. The primary bonding interaction in the resulting complex (pictured) is a donor–acceptor interaction between the filled 6s orbital on gold and the boron-based p-orbitals.
As gold and hydrogen have an isolobal relationship (i.e., they can bond in a similar way), this compound is analogous to a borohydride and is described as a boroauride. The stabilizing ligand framework means that the auride can be reversibly oxidized to an Au cation, thus giving access to auride redox chemistry in solution.
- A Molecular Boroauride: A Donor-Acceptor Complex of Anionic Gold,
Jordan W. Taylor, Alex McSkimming, Marc-Etienne Moret, W. Hill Harman,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201703235