Phosphorus-Containing Polymers
Goodyear’s 1839 discovery of the vulcanization of natural rubber obtained from rubber trees marks the beginning of the modern rubber industry. A variety of synthetic rubber products were subsequently developed. In the journal Angewandte Chemie, scientists have now introduced a new, interesting variant: a phosphorus-containing rubber with a structure that corresponds to that of natural rubber.
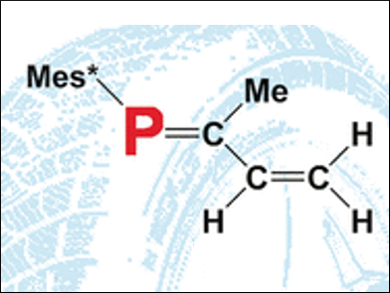
The similar properties of double bonds between carbon atoms (C=C) and phosphorus–carbon double bonds (P=C) led to the idea to try general polymerization techniques on the latter. After a number of successful attempts, researchers working with Derek P. Gates at the University of British Columbia, Vancouver, Canada, wanted to apply this concept to molecules that contain both P=C and C=C double bonds: phosphorus analogs of the building block of rubber, isoprene (2-methylbuta-1,3-diene) and its close relative, 1,3-butadiene.
Possibilities for Derivatization and Crosslinking
Starting with phosphorus-containing precursors, the team was able to synthesize the first examples of poly(1-phospha-isoprene) and poly(1-phospha-1,3-butadiene). Precise characterization with a variety of spectrometric techniques gave some insight into the molecular structures of the resulting polymers. Like in the polymerization of isoprene and related dienes (compounds with two carbon-carbon double bonds), one of the double bonds in each building block is retained. The polymerization mainly occurs through the C=C double bonds and only a tiny proportion happens at the P=C double bonds. This means that only a few phosphorus atoms are incorporated into the polymer backbone. The majority of the phosphorus atoms form side chains in which the P=C double bonds are maintained, leaving them available for further reactions or alterations to the polymers.
“Our functional phosphorus-containing materials are rare examples of polymers containing phosphaalkene moieties and offer many prospects for further derivatization and crosslinking,” according to Gates. For example, the researchers were able to bind gold ions to the polymers. “As a macromolecular ligand for gold ions, the new polymers may be of future interest in catalysis and nanochemistry. Furthermore, the successful polymerization of P=C/C=C hybrid monomers opens the door to incorporate P-functionalities into commercial rubbers such as butyl rubber or styrene-butadiene rubber that traditionally use isoprene or butadiene comonomers. Such new copolymers promise unique architectures, properties, and functionality when compared to their carbon-only analogues.”
- Polymerization of 1-Phosphaisoprene: Synthesis and Characterization of a Chemically Functional Phosphorus Version of Natural Rubber,
Klaus Dück, Benjamin W. Rawe, Michael R. Scott, Derek P. Gates,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201703590